Abstract
Chronic gastritis is a prevalent gastroentestinal disease in Korea. The purpose of this study was to investigate status of foods and nutrients intake and health related biochemical indicators in the patients with chronic gastritis. Daily food and nutrient intake, blood lipids, and antioxidant indicators in the urine, were compared between a group of 19 patients diagnosed with chronic gastritis and a control group of 27 subjects having normal gastroscopy. No significant differences were found in age, height, weight, body mass index, and blood pressure between the two groups. Daily energy intakes were 1900.6 kcal for the chronic gastritis patient group, and 1931.8 kcal for the normal control group without significant difference. No significant difference was found between the two groups in all nutrient intakes except for cholesterol. The chronic gastritis patients consumed lower amount of sugars and sweeteners but greater amount of starchy food groups such as potatoes and legumes than subjects of control group consumed. Also the chronic gastritis patients showed higher serum triglyceride concentration than the normal subjects. These results indicate that the dietary pattern of chronic gastritis patients may have relation to a change in the serum lipid level; however, more systematic research with a larger samples size is required.
Digestive diseases commonly occur in Koreans and account for 1/3 of internal diseases [1]. There are many kinds of digestive diseases which include bowel disorders, liver diseases, gallbladder and pancreatic diseases, digestive malignant, functional bowel disorders, reflux esophagitis, gastric ulcer, irritable bowel syndrome, gastric cancer, colorectal cancer. The number of Korean patients diagnosed with these diseases are continuously increasing.
Gastritis refers to an inflammation of a lining of the stomach. Gastritis may occur suddenly (acute gastritis), or it can occur slowly over time (chronic gastritis). Especially, patients of chronic gastritis have a wide range of symptoms in the gastric tissues. Main causes of chronic gastritis are infection with bacteria, primarily Helicobacter pylori, chronic bile reflux, and stress; certain diet factors such as spicy, hot, and coarse foods which irritate gastric mucosa [2,3]. In Korea, an infection of Helicobacter pyroli is highly prevalent in gastritis patients and seems to be a cause of the incidence of gastric cancer [4]. Therefore, proper treatment including taking antiacids or therapeutic diets such as avoiding hot and spicy foods are important to prevent chronic gastritis from transfering to gastric cancer.
According to previous studies long-term intake of sufficient amount of fruits and vegetables significantly reduces the risk of gastric carcinoma [5,6,7]. It is anticipated that natural antioxidants are involved in this effect in addition to other substances. Epidemiological research [8] and several meta-analyses have demonstrated that high intake of salted, pickled, or smoked foods, as well as dried fish and meat and refined carbohydrates, significantly increased the risk of developing gastric cancer while fibers, fresh vegetables, and fruits were inversely associated with gastric cancer risk [7,9,10,11,12]. However, clinical researches on dietary factor affecting gastritis in chronic gastritis patients are rarely conducted. Since Helicobacter pylori infection is a main causal factor of chronic gastritis [3], studies to investigate the dietary studies to investigate the dietary causal factors of chronic gastritis have mainly focused on the relation between Helicobacter pylori and diet [2,13,14]. Although some causal dietary factors trigger chronic gastritis, having chronic gastritis may also alter dietary intake of the patients for avoiding or alleviating the symptoms.
The purpose of this study was to investigate dietary intake status and difference of biomarkers of serum lipid and redox status in chronic gastritis patients and the healthy subjects. To achieve the goal of this study we compared daily food and nutrient intake, serum glucose and lipids, and urinary oxidative biomarkers between case patients with chronic gastritis and healthy control subjects.
Participants in this research were recruited among Korean adults living in Daecheon, Korea. The subjects agreed to provide their personal information, blood, and urine according to the purpose and the procedures of this study. Subjects who had any diseases other than chronic gastritis and who took any medicines and supplements for such diseases were excluded from this research. Nineteen subjects (11 males and 8 females) who were diagnosed with chronic gastritis using stomach endoscopy and the patient's description of their symptoms by a medical doctor, were assigned to a case group. Twenty-seven subjects (11 males and 16 females) without associated diseases of gastrointestinal tract were assigned to a control group. There was no significant difference of gender and age distribution between the two groups. The age range of each group was over thirties to seventies. This study was approved by the Institutional Review Board of Sungshin Women's University and all subjects provided written informed consent (IRB-2010-018).
Height was measured without shoes using a height stadiometer, while body weight, body fat, and lean body mass were measured using InBody (X-SCAN PLUS II, Biospace, Seoul, Korea) by well-trained research staffs. Abdominal visceral fat areas were measured by computed tomography scan (HighSpeed Advantage, General Electric Co., Evansville, IN, USA). Circumference of waist and hip were measured using a tape measure while the subject was standing up. Waist circumference was determined by measuring just above the bony landmark where one finger can fit between the iliac crest and the lowest rib, and hip circumference was determined by measuring the widest circumference of the buttocks. All measurements were repeated twice and averaged value of two-time measurement were reported. Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meters. Waist-to-hip ratio (WHR) was calculated as waist circumference divided by hip circumference.
The dietary intake survey was conducted using a 24-hr recall method through personal interviews by well-trained research staffs. Briefly, the types and amount of all foods consumed for breakfast, lunch, dinner, and snacks from rising in the morning until bedtime of the day before the survey were recorded. Food models and photographs were used to help subjects in estimating the amount of foods consumed. Daily intakes of total energy and nutrients were analyzed using Can-Pro 3.0 (The Korean Nutrition Society, Seoul, Korea).
Twelve-hr urine on the day of the dietary intake survey and 20 mL fasting venous blood after 12-hrs fasting on the following morning were collected using a urine collection bottle and vacuum blood collection tube, respectively. Serum was separated by centrifugation at × 400 g for 15 minutes. Urine and serum were stored at -70℃ until the test was conducted.
Serum glucose and lipids were analyzed using an autoanalyzer (ADVIA 1650, Bayer, Massachusetts, USA) based on enzymatic principle. In urinary oxidative biomarkers, malondialdehyde (MDA) was analyzed using HPLC (501 Waters, Massachusetts, USA), and 8-hydroxydeoxyguanosine (8-OHdG) was analyzed by microplate reader (Bio Tek, Winooski, VT, USA) with an ELISA kit. Urinary MDA and 8-OHdG were expressed as contents per g creatinine.
Data of all results were expressed as the mean and standard deviation using the SAS program (version 9.3; SAS Institute Inc., Cary, NC, USA). Differences between case and control groups were assessed using the Student's unpaired t-test. Statistical significance was set at a value of p < 0.05.
General characteristics of the patients with chronic gastritis are shown in Table 1. As shown, there was no significant difference of all characteristics including age, height, weight, BMI, and blood pressure between the two groups.
In Table 2, daily energy intakes were 1,900.6 kcal for the chronic gastritis patient group and 1,931.8 kcal for the normal control group without differences. No significant difference was found between the two groups in all nutrient intakes except cholesterol. The mean cholesterol intake of the chronic gastritis patients was 158.6 mg/day, and significantly lower than that of the normal control group (308.7 mg/day, p < 0.05). The chronic gastritis group consumed significantly less sugars than the normal control group while, they tended to consume more starchy foods, such as potatoes and legumes, than the normal control group (Table 3).
Difference of serum glucose and lipids between case and control groups is shown in Table 4. The serum level of triglycerides of the chronic gastritis group (149.3 mg/dL) was significantly higher than that of normal control group (99.2 mg/dL, p < 0.05). Nine subjects in the chronic gastritis group had a level of triglycerides higher than 150 mg/dL, however, only three subjects in the control group had a level of triglycerides higher than 150 mg/dL (data not shown).
Differences in the concentrations of urinary MDA and 8-OHdG between case and control groups are shown in Table 5. There was no significant differences of urinary oxidative biomarkers between the two groups.
We found that chronic gastritis patients consumed lower amount of cholesterol and sugars with greater amount of starchy foods than control subjects. As this study is planned as a cross-sectional design, it is difficult to determine cause-effect relationships between the dietary factors and chronic gastritis. However, it is postulated that the patients with chronic gastritis might have changed their diet for treatment or for alleviating the symptoms related to gastritis.
Moreover, food affects gastric motility and acid secretion. It is known that the concentrated carbohydrates result in stimulation of osmoreceptors and act on retardation of stomach deflation. Foods with high fat act on retardation of gastric emptying and the broths with large quantities of purine raise the acid secretion [2]. Therefore, concentrated carbohydrates and foods with high simple sugar and high fat are not recommended.
In the nutritional intake assessment, we did not find any significant differences in daily intakes of energy and nutrients in the chronic gastritis patients compared to those in the healthy control group except cholesterol intake. The daily energy intake of the chronic gastritis patient group was 1,900.6 kcal or 86.3% of Estimated Energy Requirement [15], which are not significantly different from those of the normal group. Meanwhile, the average daily cholesterol intake of the chronic gastritis group (158.6 mg) was significantly lower than that of the normal control group (308.7 mg). It is likely that low consumption of animal food such as meats, eggs, fish and shellfishes, and milk is associated with low cholesterol intake in the chronic gastritis subjects. Indeed, the total intake of animal food was lower than that of the control group (p = 0.05, data not shown). Although, cholesterol intakes of the two groups were significantly different; the mean cholesterol intake of the normal group was still in reasonable range, the level of serum cholesterol between the two groups were not significantly different.
In this study, the chronic gastritis group (149.3 mg/dL) had a significantly higher serum triglyceride level than normal control group (99.2 mg/dL). It can be postulated that high starchy foods consumption may be associated with elevated serum triglycerides in the chronic gastritis subjects. High carbohydrate consumption has been reported to cause hyperinsulinemia, postprandial hyperglycemia, and hypertriglyceridemia in South Asian adults [16]. Several epidemiological studies have also reported that low fat and high carbohydrate consumption is associated with cardiovascular disease [17,18]. A recent study [19] suggested that undesirable macronutrient composition in diet consumption may be due to excessive intake of carbohydrates and that a high sodium intake may contribute to an elevated risk of metabolic syndrome as well as cardiac dysfunction in Asian populations.
It has been reported that oxidative and inflammatory status is involved in the occurrence of various chronic diseases. Chronic gastritis is an inflammatory disease and the formation of ulcerative lesions in gastrointestinal tract may be mediated by oxygen-derived free radicals [20,21,22]. In the present study, we compared urinary MDA and 8-OHdG as oxidative biomarkers between chronic gastritis patients and the control group. Findings showed no significant difference of oxidative biomarkers between the two groups, indicating no change of oxidative status in chronic gastritis. Nevertheless, it was shown that antioxidants have a protective effect against gastritis. Kim [23] reported that administration of Portulaca oleracea, as a free radical scavenger, had a antioxidant effect and protective function against the HCl-ethanol induced gastric lesion suggesting a possibility of using a radical scavenger as medication for gastritis and gastric ulcer. Further research specifically on oxidative status and protective effects of antioxidants in gastritis patients is necessary.
Our study has a few limitations. First, as mentioned above, the cross-sectional nature of this study did not allow us to determine causal relationships among the dietary factors, biomarkers, and chronic gastritis. Second, dietary intake data estimated based on a 24-hr recall method might have not captured long-term intake patterns. Lastly, the size of the sample in both the chronic gastritis and normal control groups was not large enough to have statistical power for the results of dietary intake and several biomarkers. However, to our knowledge, this study suggests that the dietary pattern of chronic gastritis patients may be associated with a change in serum lipid level.
In this study the chronic gastritis patients consumed lower amount of cholesterol and sugars but greater amount of starchy food. They also had a higher serum triglyceride concentration compared with the normal group. These results indicate a possible relationship between a dietary pattern of chronic gastritis patients and a change in serum lipid level; however, more systematic research with a larger sample is required.
Figures and Tables
Table 1
Physical characteristics of the patients with chronic gastritis
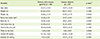
Table 2
Daily energy and nutrient intakes of the patients with chronic gastritis
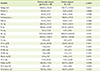
Table 3
Daily food intakes of the patients with chronic gastritis

Table 4
Serum glucose and lipid profile of the patients with chronic gastritis

References
1. Statisrics Korea. The cause of death statistics 2012. Daejeon: Statisrics Korea;2013.
2. Ddine LC, Ddine CC, Rodrigues CC, Kirsten VR, Colpo E. Factors associated with chronic gastritis in patients with presence and absence of Helicobacter pylori. Arq Bras Cir Dig. 2012; 25:96–100.

3. Makola D, Peura DA, Crowe SE. Helicobacter pylori infection and related gastrointestinal diseases. J Clin Gastroenterol. 2007; 41:548–558.

4. Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, Yim JY, Kim HU, Baik GH, Seo GS, Shin JE, Joo YE, Kim JS, Jung HC. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013; 13:104.

5. Izzotti A, Durando P, Ansaldi F, Gianiorio F, Pulliero A. Interaction between Helicobacter pylori, diet, and genetic polymorphisms as related to non-cancer diseases. Mutat Res. 2009; 667:142–157.

6. Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Duell EJ, Agudo A, Tjønneland A, Boutron-Ruault MC, Clavel-Chapelon F, Touillaud M, Teucher B, Kaaks R, Boeing H, Steffen A, Trichopoulou A, Roukos D, Karapetyan T, Palli D, Tagliabue G, Mattiello A, Tumino R, Ricceri F, Siersema PD, Numans ME, Peeters PP, Parr CL, Skeie G, Lund E, Quirós JR, Sánchez-Cantalejo E, Navarro C, Barricarte A, Dorronsoro M, Ehrnström R, Regner S, Khaw KT, Wareham N, Key TJ, Crowe FL, Blaker H, Romieu I, Riboli E. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer followup. Int J Cancer. 2012; 131:2910–2919.

7. Shimazu T, Wakai K, Tamakoshi A, Tsuji I, Tanaka K, Matsuo K, Nagata C, Mizoue T, Inoue M, Tsugane S, Sasazuki S. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Association of vegetable and fruit intake with gastric cancer risk among Japanese: a pooled analysis of four cohort studies. Ann Oncol. 2014; 25:1228–1233.

8. Dikshit RP, Mathur G, Mhatre S, Yeole BB. Epidemiological review of gastric cancer in India. Indian J Med Paediatr Oncol. 2011; 32:3–11.

9. Woo HD, Park S, Oh K, Kim HJ, Shin HR, Moon HK, Kim J. Diet and cancer risk in the Korean population: a meta-analysis. Asian Pac J Cancer Prev. 2014; 15:8509–8519.

10. Wang Q, Chen Y, Wang X, Gong G, Li G, Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer. 2014; 50:1498–1509.

11. Bertuccio P, Rosato V, Andreano A, Ferraroni M, Decarli A, Edefonti V, La Vecchia C. Dietary patterns and gastric cancer risk: a systematic review and meta-analysis. Ann Oncol. 2013; 24:1450–1458.

12. D'Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012; 31:489–498.
13. Nseir W, Mograbi J, Di Castro N, Abu-Elheja O, Abu-Rahmeh Z, Khamaysi I, Samara M, Assy N. On the association between soft drink consumption and Helicobacter pylori infection. Dig Dis Sci. 2012; 57:981–986.

14. Guo X, Zhao BH, Zhang MX. Risk factors of Helicobacter pylori infection among adults in northern China. Hepatogastroenterology. 2011; 58:306–310.
15. The Korean Nutrition Society. Dietary reference intake for Korean. Seoul: The Korean Nutrition Society;2010.
16. Misra A, Khurana L, Isharwal S, Bhardwaj S. South Asian diets and insulin resistance. Br J Nutr. 2009; 101:465–473.

17. Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006; 355:1991–2002.

18. Hu FB. Diet and cardiovascular disease prevention the need for a paradigm shift. J Am Coll Cardiol. 2007; 50:22–24.
19. Oh HY, Kim MK, Lee M, Kim YO. Macronutrient composition and sodium intake of diet are associated with risk of metabolic syndrome and hypertension in Korean women. PLoS One. 2013; 8:e78088.

20. Pihan G, Regillo C, Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987; 32:1395–1401.

21. Szelenyi I, Brune K. Possible role of oxygen free radicals in ethanolinduced gastric mucosal damage in rats. Dig Dis Sci. 1988; 33:865–871.

22. Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther. 1999; 13:Suppl 1. 13–18.

23. Kim CH. Anti-oxidant effects of Portulaca oleracea L. on HCl-ethanol induced gastritis in rats. Korean J Herbol. 2009; 24:35–40.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download