Abstract
Nutritional status of children with chronic kidney disease (CKD) is important since it affects growth and development. This study was to investigate overall diet quality measured by nutrient intake adequacy, nutrient density, and several dietary habits in children with CKD and its relationship with clinical parameters according to glomerular filtration rate (GFR). Assessment of nutritional status and diet quality was conducted in nineteen children with CKD. Average Z-scores of height, weight and body mass index (BMI) in the participants were less than standard growth rate. Nutritional status, such as Z-scores of height (p < 0.05) and serum total protein (p < 0.05), were significantly lower in the children with GFR < 75 mL/min/1.73 m2 compared to those with GFR ≥ 75 mL/min/1.73 m2. Nutrition adequacy ratio of energy, thiamin, riboflavin, vitamin B6, folate, iron, and zinc and overall diet quality were significantly poorer in the children with GFR < 75 mL/min/1.73 m2. Poorer appetite and avoidance of food were observed in the children with higher blood urea nitrogen (BUN). Intakes of iron, zinc, thiamin, niacin, and vitamin B6 were positively correlated with GFR. Intakes of calcium, potassium and folate were positively correlated with BUN, while protein intakes were negatively correlated. Overall nutrient intakes were inadequate and diet quality was decreased as kidney function was decreased. Dietary habit and appetite were also related with kidney function in this study subjects. Systemic efforts of nutritional intervention are imperative to prevent deteriorating growth and development and improve the nutritional status in children with CKD.
Nutritional status is the most important predictable factor in linear growth, development of brain and organs, and maintenance of appropriate fat and muscle stores in chronic kidney disease (CKD) [1]. It is common to cause nutritional imbalance, particularly protein-energy malnutrition in children as nutrient requirement increases due to rapid physical development, whereas body nutrient storage is low [2,3]. According to the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS), protein-energy malnutrition has been shown in 1/3 of children with CKD and it was higher in younger age group [4]. Physical manifestations of poor growth, such as low height and weight have been associated with an increased risk of mortality [1,5]. Therefore, caring for children with CKD presents unique and varied challenges for the entire healthcare team. One major challenge is balancing the nutritional requirements of these patients in order to promote appropriate growth and development with the need to control the biochemical and metabolic consequences associated with the disease progress.
Several studies have demonstrated that usual calorie intake of children with CKD was 76-88% of the recommended intake (RI) and it was one third lower than that of age-adjusted healthy children [5,6]. Other studies have shown low calorie intake (less than 90% of RI) was associated with high protein intake (more than 150% of RI), presenting the relative distribution of calories of 48% from carbohydrates, 15% from proteins, and 37% from lipids [5,7,8]. It is well-known that dietary protein should be restricted as the kidney function decreases because high protein intake deteriorates the kidney function. Other essential nutrient intake deteriorates with severity of kidney failure due to rigorous restriction of food consumption in terms of limitation in protein, potassium and sodium intake. Therefore, it would be expected that the children with CKD hardly sustain diet quality in consideration of the diversity and adequacy of food [9,10]. It is imperative to assess diet quality for children with CKD in order to reduce the risk of deficiency or unbalance in nutrient intake and to ensure overall balance of nutrient intake [11].
Although there are a number of studies to assess the nutritional status of children with CKD [2,3,8,12,13], there is lack of study to evaluate dietary quality which includes variety, adequacy, moderation, and overall balance of nutrient intakes in this sub population. In addition, although the potential impact of dietary manipulations on growth of children with CKD is of great concern, to our knowledge, few studies on diet quality in children with CKD in Korea have been reported. The purpose of this study was to evaluate the diet quality and dietary habits of children with mild to moderate CKD in an attempt to identify an early marker of deterioration in nutritional status in this population.
Nineteen children with CKD, aged from 10 to 19, were recruited between July 2010 and November 2010 at Kyung Hee Medical Center in Seoul, Korea (Table 1). Nineteen children with CKD who had renal biopsy were recruited from the University hospital. Glomerular filtration rate (GFR) (estimated by endogenous creatinine clearance calculated from serum and 24-h urine concentrations of creatinine) ranged from 9.5 to 159.6 mL/min/1.73 m2. No children had never been artificial replacement therapy and had suffered a catabolic illness for at least 3 months before the time of data collection. All children were following an ad libitum diet and were receiving medical therapy according to their clinical status. Informed consent was obtained from all parents and older children. The study protocol was approved by the Institutional Review Board (IRB) at the Kyung Hee Medical Center (IRB No: KMC IRB 1011-05).
General characteristics (age, gender, years if diagnosis, underlying disease) were investigated using constructive questionnaire. Anthropometric measurements were made with the use of standard methods for body weight and height after the subjects had been instructed to wear light clothes and not to wear shoes. These measurements were made by the same rater and the mean value of three measurements was used for data analysis. BMIs were calculated according to the following formula: BMI = weight (kg) / height2 (m2). Values for weight, height, and BMI were expressed as Z-score relative to the healthy Korean population of the same sex and chronological age according to the Korean National Growth Charts in 2007 [14,15,16]. Laboratory results included hemoglobin, hematocrit, and serum concentrations of total protein, albumin, blood urea nitrogen(BUN), creatinine, and uric acid based on the medical record.
Dietary habits were obtained by structured questionnaire, which was consisted of 6 questions including frequency of meal, frequency of breakfast, regularity of meal time, frequency of overeating, frequency of snack intake, avoidance of food, with 3-point scale [17,18]. Each questions were scored from 0 to 3, presenting that lower scores were good dietary habits. Appetite was evaluated using the Council of nutritional appetite questionnaire (CNAQ) which comprised eight items [19]. Scores for each question are summarized, resulting in total appetite score, ranging from 0 to 40. A total score less than 28 of 40 means loss of more than 5% of body weight within six months.
Dietary intake was collected by 3-day food record. All children and parents or primary care givers were carefully instructed how to record the amounts of ingested foods, snacks and beverages by household measures and were how to take the measures of the utensils before starting food recording. The food items and the amounts of food in the food records were ascertained again by a skilled clinical dietitian using food models. The same dietician analyzed all food records using the Computer Aided Nutritional Analysis program version 3.0 (The Korean Nutrition Society, Seoul, South Korea, 2010). Calorie and 15 nutrients (protein, vitamin A, B1, B2, B6, C, E, niacin, folate, Na, K, Ca, P, Fe, and Zn) from the food records were determined for nutrient adequacy ratio (NAR) estimation, which was calculated as the actual intake of a nutrient divided by the Dietary Recommended Intakes for Korean (KDRIs) for chronological age. Mean adequacy ratio (MAR) was calculated as a measure of overall nutrient adequacy, where MAR is the sum of each NAR divided by the number of nutrients [20]. To estimate MAR, each value of NAR was truncated at 1.0 so that a nutrient with a high NAR could not compensate for a nutrient with a low NAR [21,22]. For both NAR and MAR a value of 1.0 indicates that the intake of all nutrients is equal to the recommended intakes, and a MAR below 1.0 indicates lower than the recommended intake for one or more nutrients [23].
Index of nutritional quality (INQ) was used for evaluation of nutrient allowance per 1,000 kcal [24]. To determine a value of INQ for each nutrient, recommended intakes of each nutrient was converted into an allowance per 1,000 kcal. After that, actual intake of each nutrient per 1,000 kcal of actual calorie intake was divided by each recommended intake per 1,000 kcal.
Statistical analysis was performed using Statistical Analysis System (SAS) version 9.1. Results were presented as mean ± SD. Student's t-test were used to compare the means of the groups. The correlation between clinical parameters related to renal function and diet was tested by Spearman's correlation coefficient. Statistical significance was defined as p < 0.05.
Nutritional status of the patients is shown in Table 2. Patients had Z-scores of height (-0.2 ± 0.8), weight (-0.2 ± 1.3) and BMI (-0.2 ± 1.3) below those for the healthy population. Z-score of height deteriorated as GFR decreased, indicating significant decrease in the patients who with GFR < 75 mL/min/1.73 m2 (0.2 ± 0.7) compared to those who with GFR ≥ 75 mL/min/1.73 m2 (- 0.7 ± 0.7) (p < 0.05). All biochemistry results were within normal range except for BUN (24.0 ± 23.1 mg/dL) and creatinine (1.7 ± 1.8 mg/dL). Hematocrit (p < 0.05), total protein (p < 0.05), and calcium (p < 0.05) were significantly lower in GFR < 75 mL/min/1.73 m2 group than in GFR ≥ 75 mL/min/1.73 m2 group. However, BUN (p < 0.01), creatinine (p < 0.01), and uric acid (p < 0.01) were significantly higher in in GFR < 75 mL/min/1.73 m2 group than in GFR ≥ 75 mL/min/1.73 m2 group.
Eating habits and appetite in children with CKD were not significantly different between two groups (data not shown). Mean intakes of energy in the patients with CKD were about 70% of the recommended intakes (Table 3). Energy intakes in the patients with lower GFR (26.6 ± 9.3 kcal/kg of body weight (BW)/day, 60% of the recommendation) were significantly less than in those with higher GFR (37.4 ± 11.1 kcal/kg of BW/day, 80 % of the recommendation) (p < 0.001). Adequacy of dietary energy intake was only 70% (0.7 of NAR) of the recommended amounts in this study subjects and it significantly decreased in lower GFR groups (p < 0.001). Overall nutrient intakes were less than the recommended amounts except for protein (1.0 of NAR) and sodium (1.0 of NAR). Thiamin (p < 0.001), riboflavin (p < 0.001), vitamin B6 (p < 0.01), folate (p < 0.05), iron (p < 0.01) and zinc (p < 0.01) were in the patients with lower GFR group significantly lower compared to those who with GFR ≥ 75 mL/min/1.73 m2. Overall nutrient adequacy presented by mean adequacy ratio (MAR) was 0.7 ± 0.1, which means the subjects obtained less than 75% of nutrients of recommended amounts from usual diet. MAR was also significantly lower in the lower GFR group (p < 0.01).
Nutrient density, expressed in INQ, appeared to be around 1, which means that the consumption of each nutrient per 1,000 kcal satisfied individual's requirement for each nutrient. It can be postulated that reduced overall nutrient intakes might be due to low calorie intakes. However, INQ of some nutrients, such as folate (0.6 ± 0.2), vitamin C (0.7 ± 0.5), calcium (0.6 ± 0.2) and potassium (0.3 ± 0.1) were appeared to be considerably low, indicating low in absolute amounts of these nutrients in their diet, regardless of calorie intakes. Minerals such as calcium (p < 0.05), phosphorus (p < 0.01), and potassium (p < 0.05) were significantly higher in lower GFR group than in higher GFR group, while sodium intakes (p < 0.05) were significantly higher than in lower GFR group than in higher GFR group.
Avoidance of food (r = -0.61, p < 0.01) and total scores of appetite (r = 0.59, p < 0.01) were correlated significantly with levels of BUN (Table 4). GFR correlated positively with dietary intake of iron (r = 0.53, p < 0.05), zinc (r = 0.46, p < 0.05), thiamin (r = 0.59, p < 0.01), niacin (r = 0.48, p < 0.05), and vitamin B6 (r = 0.50, p < 0.05). BUN correlated positively with protein (r = 0.51, p < 0.05) and negatively with calcium (r = -0.59, p < 0.05), potassium (r = -0.63, p < 0.01) and folate (r = -0.55, p < 0.05). No significant correlations were found between creatinine and dietary nutrient intakes.
The purpose of this study was to evaluate the diet quality of children with mild to moderate CKD in an attempt to identify an early marker of deterioration in nutritional status in this population. Overall nutrient intakes were inadequate and diet quality was decreased as kidney function was decreased. Dietary habit and appetite were also related with kidney function in this study subjects.
Anthropometric parameters, which are commonly used for nutritional assessment in children, reflect children's growth and development [24,25]. In the study of Nydegger et al [26]. nutritional status of the children with end-stage renal failure were assessed based on anthropometry and compared nutritional status with the children in pre-dialysis stage. In that study, no significant differences between the children with GFR < 25 mL/min/1.73 m2 and those with GFR ≥ 75 mL/min/1.73 m2 were shown in Z-scores of height, body weight and BMI. In another study, however, Z-scores of height and body weight in the children with GFR below 25 mL/min/1.73 m2 were significantly lower compared to those with normal kidney function [27]. Our study demonstrated that Z-score of height in the group of GFR < 75 mL/min/1.73 m2 was lower than in the group of GFR ≥ 75 mL/min/1.73 m2, while the Z-scores of body weight and BMI between two groups were not different. The differences of each study results were caused by the variation of study design, which were related to the participant's eligibility including clinical manifestation and treatment stauts [3,28].
Serum BUN, creatinine, and uric acid levels start to increase when kidney function decreases below 75% and metabolic disturbances in calcium and phosphorus definitely appears when kidney function decreases below 50% [29,30]. In the study of Norman et al. [27], serum phosphorus levels in the children with GFR below 25 mL/min/1.73 m2 were higher compared to those with GFR above 25 mL/min/1.73 m2, but serum calcium levels were not different between these two groups. Another study demonstrated that serum calcium and phosphorus levels were not different between the groups of GFR less and above 50 mL/min/1.73 m2 [31]. In this study, serum calcium levels in the group of GFR below 75 mL/min/1.73 m2 was lower compared to the group of GFR above 75 mL/min/1.73 m2. It is considered that the each study result might be different according to the assignment of the participants into GFR group.
It has been reported that the children with CKD has poor appetite and dietary restriction and thus they consumed less calories and essential nutrients compared to those with normal kidney function [9,26,32,33]. It has been also reported that rigorous dietary restriction leads less intakes of sodium, potassium, phosphorus, vitamin C and B6, and folate [34]. Dietary energy and protein intakes for pre-dialysis children are recommended as much as for the children with normal kidney function, and intakes of sodium and potassium are recommended considering the degree of swelling and the level of serum level [34]. According to this study result of dietary intakes in the children with CKD, most of the essential nutrients in the group of GFR below 75 mL/min/1.73 m2 were less than the recommended amount. However, sodium intakes in all children with CKD were about 200% of recommendation. It is imperative that dietary intervention would be required for reduction of sodium intake.
This study demonstrated that dietary adequacy for calories and some nutrients including thiamin, riboflavin, vitamin B6, folate, iron and zinc were poorer in the group of GFR below 75 mL/min/1.73 m2 compared to the group of GFR above 75 mL/min/1.73 m2. INQ score, which is useful to estimate the individual nutrient intakes adjusted for total calorie intakes [35], of calcium, phosphorus, and potassium were higher in the group of GFR below 75 mL/min/1.73 m2, indicating higher nutritional quality. However, some limitations have been pointed out in the previous literature that INQ was not available for the dietary quality assessment in whom with severely diminished total calorie intakes [35]. Therefore, nutritional quality may be overestimated in the children with GFR below 75 mL/min/1.73 m2 who consumed dietary energy 56.7% of the recommendation. Meanwhile, NAR of thiamin, riboflavin, vitamin B6, folate, iron and zinc in the group of GFR below 75 mL/min/1.73 m2 were ranged from 0.3 to 0.7, presenting severe nutritional unbalance and inadequacy.
A diminished appetite, also known as anorexia, is a frequent complication of uremic syndrome in CKD patients [35,36,37,38]. A diminished appetite due to uremic syndrome decreases food consumption and increases energy expenditure [39]. It can be a cause of malnutrition and growth retardation in children and consequently leads physical, emotional and social stress [11]. Considering that the aim of nutritional assessment is to identify patients at potential or substantial risk of malnutrition, nutritional assessment has necessarily to be feasible, easy-to-use, cost-effective, and non-invasive. Although the tools or scales for evaluating dietary habits and appetite differed among the studies, dietary habits and appetite has been widely used as surrogate indicators for nutritional status in diverse population [19,40,41,42]. In this study, dietary habits, especially avoidance of food, and anorexia in children with CKD deteriorated as BUN level increased. This study result presented that dietary habits and appetite may be significantly affected by kidney function and suggested dietary habits and appetite were easy-to-use indicators for assessing nutritional status.
Due to a small number of study subjects, it may be difficult to generalize study results. Considering we satisfied the power of sample size according to the literature review, however, this study results are meaningful to explain the characteristics of children with CKD. In addition, there is little evidence to describe the diet quality and dietary habits of this population, although the prevalence rate of pediatric CKD in Korea is 3.68 per million which is higher than the prevalence in other countries (e.g., 14.6 per million in USA). Therefore, this study provides preliminary evidences for future investigations related to dietary intervention for children with CKD. A further study to investigate the effect of comprehensive dietary intervention on dietary intakes and consequently on nutritional status in children with CKD are warranted.
Overall nutrient intakes were inadequate and diet quality was decreased as kidney function was decreased. This study suggested that dietary habit and appetite were early predictable indicators for nutrient intakes and nutritional status during CKD presenting dietary habits and appetite were related with kidney function in this study subjects. Systemic efforts of nutritional intervention, especially increasing appetite and balancing dietary intake, are imperative to prevent deteriorating growth and development and improve the nutritional status in children with CKD.
Figures and Tables
Table 2
Nutritional status in children with chronic kidney disease
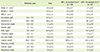
Values are presented as mean ± SD unless otherwise indicated.
BMI: body mass index = weight (kg) / height (m2), BUN: blood urea nitrogen
*Z - score = [(χ/M)L - 1] / LS (χ was from height, weight and BMI for calculation of Z-score, respectively, M: Median, L: Box-cox power, S: Coefficient of variation); †p < 0.05; ‡p < 0.01.
References
1. Graf L, Candelaria S, Doyle M, Kaskel F. Nutrition assessment and hormonal influences on body composition in children with chronic kidney disease. Adv Chronic Kidney Dis. 2007; 14:215–223.

2. Foster BJ, Leonard MB. Nutrition in children with kidney disease: Pitfalls of popular assessment methods. Perit Dial Int. 2005; 25:Suppl 3. S143–S146.

3. Kim YH. Pediatric medical nutrition. Seoul: Korea medical book publisher Co.;2007.
4. Mahan JD, Warady BA. Consensus Committee. Assessment and treatment of short stature in pediatric patients with chronic kidney disease: a consensus statement. Pediatr Nephrol. 2006; 21:917–930.

5. Rees L, Shaw V. Nutrition in children with CRF and on dialysis. Pediatr Nephrol. 2007; 22:1689–1702.

6. Lee JW, Lee MS, Kim JH, Son SM, Lee BS. Nutritional assessment. Paju: Kyomunsa;2007.
7. Rosenkranz J, Bonzel KE, Bulla M, Michalk D, Offner G, Reichwald-Llugger E, Schärer K. Psychosocial adaptation of children and adolescents with chronic renal failure. Pediatr Nephrol. 1992; 6:459–463.

8. Rashid R, Neill E, Smith W, King D, Beattie TJ, Murphy A, Ramage IJ, Maxwell H, Ahmed SF. Body composition and nutritional intake in children with chronic kidney disease. Pediatr Nephrol. 2006; 21:1730–1738.

9. KDOQI Work Group. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009; 53:S11–S104.
10. Beto JA, Bansal VK. Medical nutrition therapy in chronic kidney failure: integrating clinical practice guidelines. J Am Diet Assoc. 2004; 104:404–409.

11. Sin EK, Lee YK. Evaluation of food and nutrient intake of preschool children in day-care centers. J Korean Soc Food Sci Nutr. 2005; 34:1008–1017.
12. Norman LJ, Macdonald IA, Watson AR. Optimising nutrition in chronic renal insufficiency--growth. Pediatr Nephrol. 2004; 19:1245–1252.

13. Sahpazova E, Kuzmanovska D, Todorovska L, Bogdanovska A. Nutritional status, protein intake and progression of renal failure in children. Pediatr Nephrol. 2006; 21:1879–1883.

14. Lee JH, Lee BS, Kang HG, Hahn HW, Ha IS, Cheong HI, Choi Y, Kim SJ. Analysis of factors affecting height growth after transplantation in children. J Korean Soc Pediatr Nephrol. 2000; 4:84–91.
15. Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, Kim YT, Lee CG. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008; 51:1–25.

16. Garza C, de Onis M. Rationale for developing a new international growth reference. Food Nutr Bull. 2004; 25:S5–S14.

17. Han MJ, Cho HA. The food habit and stress scores of high school students in Seoul area. Korean J Food Cookery Sci. 2000; 16:84–90.
18. Choi MJ, Yoon J. The effect of eating habits and nutrient intake on the physical growth indices in preschool children. Korean J Community Nutr. 2003; 8:3–14.
19. Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, Diebold MR, Morley JE. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005; 82:1074–1081.

20. Hatløy A, Torheim LE, Oshaug A. Food variety--a good indicator of nutritional adequacy of the diet? A case study from an urban area in Mali, West Africa. Eur J Clin Nutr. 1998; 52:891–898.

21. Guthrie HA, Scheer JC. Validity of a dietary score for assessing nutrient adequacy. J Am Diet Assoc. 1981; 78:240–245.

22. Ries CP, Daehler JL. Evaluation of the Nutrient Guide as a dietary assessment tool. J Am Diet Assoc. 1986; 86:228–233.

23. Coles GA, Peters DK, Jones JH. Albumin metabolism in chronic renal failure. Clin Sci. 1970; 39:423–435.

24. Hansen RG, Wyse BW. Expression of nutrient allowances per 1,000 kilocalories. J Am Diet Assoc. 1980; 76:223–227.

25. Mascarenhas MR, Meyers R, Konek S. Outpatient nutrition management of the neurologically impaired child. Nutr Clin Pract. 2008; 23:597–607.

26. Nydegger A, Strauss BJ, Heine RG, Asmaningsih N, Jones CL, Bines JE. Body composition of children with chronic and end-stage renal failure. J Paediatr Child Health. 2007; 43:740–745.

27. Norman LJ, Coleman JE, Macdonald IA, Tomsett AM, Watson AR. Nutrition and growth in relation to severity of renal disease in children. Pediatr Nephrol. 2000; 15:259–265.

28. Chan JC, Williams DM, Roth KS. Kidney failure in infants and children. Pediatr Rev. 2002; 23:47–60.

29. Fogo A, Kon V. Pathophysiology of progressive renal disease. In : Barratt TM, Avner ED, Harmon WE, editors. Pediatric nephrology. 4th ed. Baltimore (MA): Lippincott Williams & Wilkins Co;1999. p. 1183–1196.
30. Yoo KH. Chronic renal failure in children. J Korean Pediatr Soc. 2003; 46:430–433.
31. Orejas G, Santos F, Málaga S, Rey C, Cobo A, Simarro M. Nutritional status of children with moderate chronic renal failure. Pediatr Nephrol. 1995; 9:52–56.

32. Betts PR, Magrath G. Growth pattern and dietary intake of children with chronic renal insufficiency. Br Med J. 1974; 2:189–193.

33. Abitbol CL, Warady BA, Massie MD, Baluarte HJ, Fleischman LE, Geary DF, Kaiser BA, McEnery PT, Chan JC. Linear growth and anthropometric and nutritional measurements in children with mild to moderate renal insufficiency: a report of the Growth Failure in Children with Renal Diseases Study. J Pediatr. 1990; 116:S46–S54.

34. Quan A, Baum M. Protein losses in children on continuous cycler peritoneal dialysis. Pediatr Nephrol. 1996; 10:728–731.

35. Oh SY. Analysis of methods on dietary quality assessment. Korean J Community Nutr. 2000; 5:362–367.
36. Ikizler TA, Greene JH, Wingard RL, Parker RA, Hakim RM. Spontaneous dietary protein intake during progression of chronic renal failure. J Am Soc Nephrol. 1995; 6:1386–1391.

37. Bergström J. Regulation of appetite in chronic renal failure. Miner Electrolyte Metab. 1999; 25:291–297.

38. Kopple JD, Greene T, Chumlea WC, Hollinger D, Maroni BJ, Merrill D, Scherch LK, Schulman G, Wang SR, Zimmer GS. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int. 2000; 57:1688–1703.

39. Mak RH, Cheung W, Cone RD, Marks DL. Leptin and inflammation-associated cachexia in chronic kidney disease. Kidney Int. 2006; 69:794–797.

40. Dwyer JT, Larive B, Leung J, Rocco M, Burrowes JD, Chumlea WC, Frydrych A, Kusek JW, Uhlin L. Hemodialysis Study Group. Nutritional status affects quality of life in Hemodialysis (HEMO) Study patients at baseline. J Ren Nutr. 2002; 12:213–223.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download