Abstract
Recent studies have suggested that natural agents such as isoflavones, resveratrol, and anthocyanin have beneficial effects on metabolic syndrome-related disorders. The objective of this study was to evaluate dietary isoflavone intake, urinary isoflavone level, and their relationship with the risk of metabolic syndrome (MetS) among Korean postmenopausal women. The subjects included 46 MetS and 60 controls. The MetS risk score was determined by adding the number of risk factors such as waist circumference, blood pressure (BP) and levels of triglyceride (TG), HDL-cholesterol, and glucose. Dietary isoflavone intake was not significantly different between the MetS and control groups; however, the urinary daidzein level was significantly higher in the MetS subjects compared to that of the controls. Subjects with high TG had higher urinary daidzein and isoflavone (daidzein + genistein) levels than those without such abnormalities. But, the MetS risk score showed no significant correlation with urinary daidzein, genistein, and isoflavone excretions.
Metabolic syndrome (MetS) describes the clustering of abdominal obesity, lipid abnormalities, hypertension, and hyperglycemia. The prevalence of MetS is increasing worldwide, and this sharp increase has also been observed in Asian countries [1]. According to data from the Korean National Health and Nutrition Examination Survey (KNHANES) 1998-2007, the age-adjusted prevalence of MetS increased significantly from 24.9% in 1998, 29.2% in 2001, and 30.4% in 2005 to 31.3% in 2007 [2].
In South Korea, the prevalence of MetS in men was higher than in women aged 20-49 years, but the prevalence of MetS in women increased significantly at age 50 years and surpassed men afterward [2]. Considering that this noticeable increase in MetS was observed for women starting at the age of 50, it seems likely that menopause might be a reason for this change. Postmenopausal women are considered to have a higher risk of coronary artery disease and osteoporosis than premenopausal women [3]. During menopause, the pattern of hormone secretion changes and causes fat accumulation in visceral adipose tissues of the abdomen [4]. In Heidari and colleagues' study, the prevalence of MetS in the postmenopausal women increased significantly in comparison with that during menopause and the pre-menopause period, even after age adjustment [5].
Many previous studies have reported that hormone replacement therapy (HRT) in postmenopausal women has beneficial effects on lipids profiles, blood pressure, and glucose homeostasis [6]. Estrogens positively affect lipid and lipoprotein metabolism, increase production of nitric oxide, and decrease production of endothelin [7]. But, some studies indicated that HRT is not effective in decreasing the risk for CVD prevalence [8,9].
Some recent studies have suggested that natural agents such as isoflavones, resveratrol, and quercetin have beneficial effects on MetS-related disorders [10,11]. Especially isoflavones, a group of biologically active compounds found in soybeans and other legumes, bind to estrogen receptor sites and have weak estrogenic effects [12,13]. In a cross-sectional study, usual dietary isoflavone intake in postmenopausal women had a protective role against CVD [14]. And, in a randomized controlled trial (RCT) study, it was reported that genistein supplementation in postmenopausal women improved glycemic and vascular reactivity indexes in normoinsulinemic patients, while improving the insulin sensitivity indexes in hyperinsulinemic patients [15]. Meta-analysis of RCTs reported that isoflavone use was not related to a significant glycemia reduction in postmenopausal non-Asian women [16]. Like this, the effect of isoflavone intake on MetS-related disorders (CVD and regulation of blood glucose) is not consistent with the study conducted on humans. Thus, the purpose of this study is to observe the relationship of the nutritive conditions of isoflavones and the factors related to metabolic syndrome in postmenopausaul women who are exposed to various metabolic risks.
The study involved 106 women aged 47 to 85 years. Study subjects were recruited for the study by the use of advertisements. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Sookmyung Women's University Research Ethics Committee. After sufficiently explaining the objective and details of this research, written informed consent was obtained from participants.
Each participant was interviewed by the investigators using a questionnaire. The questionnaire elicited information about the participant's age, cigarette smoking, alcohol consumption, and daily physical activity level. Dietary intakes were surveyed under investigator guidance using a 24-h recall method. A color photograph of the food items, dishes, glasses, and spoons of various sizes were used to improve the accuracy of portion size estimates. The 24-h recall results were analyzed by a computer-aided nutrient analysis program for professionals (CAN-Pro 2.0, APAC Intelligence, Seoul, South Korea). To calculate the intake of isoflavones, domestic and overseas data analyzing genistein, and diadzein, the major isoflavones, were used [17-20].
Height and body weight were measured by using an electrical digital scale (DS-102; JENIX, Korea) while the subjects wearing light clothes without shoes were in the standing position. Body weight was measured to the nearest 0.1 kg and height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as body weight (kg) / [height (m)]2. The percentage of body fat was calculated by bioelectric impedance analysis using InBody 3.0 (Biospace, Seoul, South Korea). The waist circumference was measured at the smallest circumference between the rib cage and iliac crest with the subject in the standing position. Systolic blood pressures (SBP) and diastolic blood pressures (DBP) were measured twice with an automatic electronic sphygmomanometer (BP-750A; NISSEI Commerce, Ltd., Tokyo, Japan).
Blood samples were collected from the subjects on empty stomachs. The samples were left at room temperature for 30 minutes, centrifuged for 15 minutes at 2,500 rpm to separate the serum, and then stored at -70℃. Fasting glucose, triglyceride (TG), and high density lipoprotein-cholesterol (HDL-cholesterol) were determined until an auto analyzer (Hitachi 747; Hitachi, Tokyo, Japan). Urine samples were taken and kept frozen at -20℃ until they were analyzed. Urinary creatinine concentrations were measured with a Hitachi chemistry auto analyzer (Hitachi 747; Hitachi, Tokyo, Japan) by using a kit from Boehringer Mannheim (Mannheim, Germany) that is based on a kinetic modification of the Jaffe's reaction.
Analysis of isoflavone in urine was performed using the method of Maskarinec et al. [21] which is based on the HPLC method. Genistein and diadzein in urine were analyzed by using LC-10AVP (Shimadzu, Kyoto, Japan) for HPLC, Shimpack VP-ODS (250 × 4.6 mm, 4 um) reverse-phase column (Shimadzu, Kyoto, Japan), and Shim pack (10 × 4.6 mm, 4 um) direct connect guard column (Shimadzu, Kyoto, Japan). Then genistein and diadzein value in urine changed into creatinine value (nmol/mg Cr). The total amount of isoflavone in urine was calculated by adding genisteina and diadzein in urine.
The presence of MetS was defined using a modified version of the ATP III criteria [22]. The five MetS components were defined using the following ATP III categorizations: 1) abdominal obesity (defined as waist circumference ≥ 85 cm for women in Korean) [23], 2) high blood pressure (≥130 mmHg SBP and/or ≥85 mmHg DBP), 3) high fasting blood glucose (≥100 mg/dL), 4) high TG (≥150 mg/dL), and 5) low HDL-cholesterol (<50 mg/dL). The third criterion, fasting blood glucose, was changed to 100 mg/dL from the initial cutoff point of 110 mg/dL according to the revised guideline [24]. The subjects with three or more of the above mentioned criteria were defined as having MetS.
MetS risk score was determined by adding the number of risk factors, waist circumference, TG, HDL-cholesterol, glucose, and blood pressure. The study population was divided into two groups according to the MetS risk score: control group (MetS risk score < 3; n = 60), and MetS group (MetS risk score ≥ 3; n = 46).
The statistic analyses were performed using the SAS 9.2 program (SAS Inc., Cary, NC, USA). Differences in continuous variables of socio-demographics, anthropometric, clinical, and metabolic parameters between the MetS group and the control group were evaluated by the t-test for normally distributed variables. As the distributions of variables deviated significantly from normal, they were normalized by log-transformation, and log-transformed values were used in the analysis. Differences in categorical socio-demographic variables were evaluated by the chi-square test. Correlations between urinary isoflavone excretion and MetS risk factor variables were assessed by Pearson's partial correlation coefficients, adjusting for age and energy intake. All of the above analyses were considered significant at p < 0.05 (two tailed).
Socio-demographic, anthropometric, and metabolic parameters of the subjects in the MetS group and control group are shown in Table 1. The average age of the control and MetS group participants were 62.37 yrs and 63.46 yrs, respectively, and there was no significant difference. Body weight (p < 0.05), BMI (p < 0.001) and percent body fat (p < 0.001) of the MetS group were significantly higher than those of the control group. In metabolic parameters, the MetS group exhibited significantly higher waist circumference (p < 0.0001), SBP (p < 0.001), DBP (p < 0.01), TG (p < 0.0001), and glucose (p < 0.001) levels and lower HDL-cholesterol level (p < 0.0001) than the control group. There were no differences between the MetS group and the control group according to alcohol use, smoking status, and physical activity.
Energy and nutrient intakes of the MetS group and control group are shown in Table 2. The average energy intakes of the control and MetS group were 1374.31 kcal and 1393.34 kcal, respectively, with no significant difference. Also, no significant differences in nutrient intakes between the MetS group and the control group were observed. Food intakes from each food group of the MetS group and control group are shown in Table 3. The average food intakes of the control and MetS group were 918.91 g and 923.89 g, respectively, with no significant difference. Also, there was no significant difference in food intake from each group between two groups.
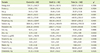
Isoflavone intakes and excretion in urine of the MetS group and control group are shown in Table 4. The average isoflavone intakes of the control and MetS group were 31.64 mg/day and 27.99 mg/day, respectively, and there was no significant difference. The MetS group showed a significantly higher daidzein level in urine (p < 0.05) than the control group. Also, the MetS group tended to be high in urinary isoflavone level compared to the control group without statistical significance (p = 0.0583).
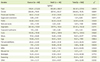
Urinary isoflavone excretion according to the abnormalities of MetS individual diagnostic components is presented in Table 5. Subjects with high serum TG had higher urinary daidzein (p < 0.05), genistein (p < 0.01) and isoflavone (p < 0.05) excretions than those without such abnormalities.
Pearson's partial correlation coefficients among the MetS risk score, diagnostic components of MetS, and urinary isoflavone excretions are shown in Table 6. The correlation analysis indicated that the urinary genistein excretion (r = 0.238, p < 0.05) had positive correlation with the serum TG level after adjust ments for age and energy intake. The MetS risk score had no significant correlation with urinary daidzein, genistein, and isoflavone excretions.
In this cross-sectional study, no significant difference was observed in daily dietary isoflavone consumption between the Korean postmenopausal women with and without MetS. Also, urinary isoflavone excretion was found to be positively associated with TG level, and inversely associated with HDL-cholesterol levels.
In this study, we evaluated and compared isoflavone intake in Korean postmenopausal women between the MetS and control groups. The daily isoflavone intakes by the control group and the MetS group were 31.64 mg and 27.99 mg, respectively, with no significant difference. In the Shanghai Women's Health Study (SWHS), the dietary isoflavone intake in women aged 40-70 years was 30.52 mg/day [25], and the isoflavone intake in Japanese postmenopausal women was 26.7 mg/day [26]. However, in the Framingham Offspring Study, the daily isoflavone intake in postmenopausal women was 0.779 mg. Compared to Asian countries where soy food intake is high, intake of isoflavone was low in western countries such as the US [27]. Postmenopausal Korean women showed a similar level of isoflavone intake compared to that of Chinese and Japanese middle aged women.
In the Framingham Offspring Study, de Kleijn et al. [27] studied the relation of phytoestrogen intake and MetS in 939 postmenopausal women and reported that the TG levels in the highest quartile category of intake for isoflavones (sum of daidzein, genistein, formononetin, biochanin A and coumestrol) was significantly lower than that in the lowest quartile category of intake for isoflavones. Also, in the highest quartile of isoflavone intake, the mean cardiovascular risk factor metabolic score was significantly lower than that in the lowest quartile. However, in some intervention studies performed on postmenopausal women, it was reported that supplementing isoflavones did not have any effect in improving serum lipid [28,29], and making it difficult to reach a consistent conclusion on the positive effect that isoflavone intake has on MetS.
Meanwhile, our study has some limitations in calculating isoflavone intake. It should first be mentioned that in our study, daidzein and genistein intakes were calculated by using published databases, but the nutrient data are derived from the analysis of food samples from various sources and, as such, have limited accuracy then applied in a particular population [30]. Such limitation likely reduced the ability to detect significant associations in this study. Second, there is the possibility that this study did not reflect the accurate intake of isoflavones, because the intake of isoflavones was calculated by adding only daidzein and genistein.
Urinary levels of isoflavonoid excretions are aggregate measurements of amounts of consumption, metabolism, and absorption of dietary isoflavone [31-34]. The dietary intake of daidzein and genistein after adjusting for total energy intake was significantly correlated with the urinary excretion [35]. When metabolized by gut flora, daidzein is converted to dihydrodaidzein, O-desmethylangolensin (O-DMA), and equol, while genistein is metabolized to dihydrogenistein [31]. Both precursors and metabolites can be absorbed into the blood and then excreted, mainly in urine [36].
In our study, the Mets group had a significantly higher urinary daidzein level than the control group. And the urinary daidzein excretion was positively correlated with the serum TG level after adjustment for age and energy intake. These results are contrary to what we expected. We speculate that people consuming high isoflavones may have a certain dietary pattern related to developing Mets or increasing serum TG. But further studies are needed to prove this speculation.
This study found no meaningful relationship between the intake of isoflavones and the isoflavone level in urine, which was similar after adjusting for BMI and daily energy intake amount (data now shown). Referring to other studies, metabolic activity of isoflavones may differ by individual [37]. Besides the intake amount of isoflavone, various factors which affect the excretion amount of isoflavones have been reported. In middle-aged and elderly Chinese women, it was reported that urinary excretion of isoflavones was correlated with soy food intake and healthy lifestyle (e.g., regular exercise) but was inversely associated with fruit intake [25]. Also, Wu and colleagues' study showed that urinary dihydrodaidzein, equol, dihydrogenistein, and the metabolites of daidzein and genistein had a significantly negative correlation with BMI [25]. The metabolization of daidzein occurs in the gut through the action of a particular class of bacteria Clostridium sp, Eubacterium ramulus, Bacteroides ovatus, Bifidobacterium breve, etc [36]. The gut bacterial flora in obese individuals is known to differ from that in non-obese individuals. Like this urinary isoflavone excretion is affected by various factors such as weight, lifestyle, food intake and colonic environment. Thus, it is difficult to assess isoflavone exposure status just by isoflavone in urine, so analysis of various indicators which can reflect the actual isoflavone exposure (e.g., serum level, etc) is necessary in the following study.
In this study, the MetS group did not show a significant difference in isoflavone intake compared to the control group, but the MetS group showed a higher urinary isoflavone. Also, urinary daidzein was positively correlated with serum TG in the Korean postmenopausal women after adjusting for age and energy intake. There are some studies reporting the effect of isoflavone intake on MetS such as obesity, high blood pressure, hyperlipidemia, and impaired glucose metabolism, while there are no studies that observed the relationship between MetS and isoflavone excretion in urine, and this may elevate the value of this study.
Figures and Tables
Table 5
Urinary isoflavone excretions according to the abnormalities in MetS components*

BP: blood pressure, HDL-C: high density lipoprotein-cholesterol, TG: triglyceride, p: Significance as determined by Student's t-test (presence vs. absence of abnormalities in MetS components).
*Values are presented as mean ± SD; †Waist circumference, ≥85 cm; ‡Systolic/diastolic BP ≥130/85 mmHg; §<50 mg/dL; ∥≥150 mg/dL; ¶≥100 mg/dL.
References
1. Nestel P, Lyu R, Low LP, Sheu WH, Nitiyanant W, Saito I, Tan CE. Metabolic syndrome: recent prevalence in East and Southeast Asian populations. Asia Pac J Clin Nutr. 2007; 16:362–367.
2. Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, Choi SH, Cho SI, Park KS, Lee HK, Jang HC, Koh KK. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011; 34:1323–1328.
3. Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989; 321:641–646.

4. Poehlman ET. Menopause, energy expenditure, and body composition. Acta Obstet Gynecol Scand. 2002; 81:603–611.

5. Heidari R, Sadeghi M, Talaei M, Rabiei K, Mohammadifard N, Sarrafzadegan N. Metabolic syndrome in menopausal transition: Isfahan Healthy Heart Program, a population based study. Diabetol Metab Syndr. 2010; 2:59.

6. Schneider JG, Tompkins C, Blumenthal RS, Mora S. The metabolic syndrome in women. Cardiol Rev. 2006; 14:286–291.

7. Bracamonte MP, Miller VM. Vascular effects of estrogens: arterial protection versus venous thrombotic risk. Trends Endocrinol Metab. 2001; 12:204–209.

8. Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002; 288:49–57.

9. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing Group for the Wome's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002; 288:321–333.

10. Xia X, Weng J. Targeting metabolic syndrome: candidate natural agents. J Diabetes. 2010; 2:243–249.

11. Cherniack EP. Polyphenols: planting the seeds of treatment for the metabolic syndrome. Nutrition. 2011; 27:617–623.

12. Potter SM. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J Nutr. 1995; 125:606S–611S.
13. Kui per GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998; 139:4252–4263.

14. Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr. 2001; 131:1202–1206.

15. Villa P, Costantini B, Suriano R, Perri C, Macrì F, Ricciardi L, Panunzi S, Lanzone A. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: relationship with the metabolic status. J Clin Endocrinol Metab. 2009; 94:552–558.

16. Ricci E, Cipriani S, Chiaffarino F, Malvezzi M, Parazzini F. Effects of soy isoflavones and genistein on glucose metabolism in perimenopausal and postmenopausal non-Asian women: a meta-analysis of randomized controlled trials. Menopause. 2010; 17:1080–1086.

17. de Kleijn MJ, van der Schouw YT, Wilson PW, Adlercreutz H, Mazur W, Grobbee DE, Jacques PF. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study(1-4). J Nutr. 2001; 131:1826–1832.

18. Franke AA, Hankin JH, Yu MC, Maskarinec G, Low SH, Custer LJ. Isoflavone levels in soy foods consumed by multiethnic populations in Singapore and Hawaii. J Agric Food Chem. 1999; 47:977–986.

19. Liggins J, Bluck LJ, Runswick S, Atkinson C, Coward WA, Bingham SA. Daidzein and genistein content of fruits and nuts. J Nutr Biochem. 2000; 11:326–331.

20. Lee SK, Lee MJ, Yoon S, Kwon DJ. Estimated isoflavone intake from soy products in Korean middle-aged women. J Korean Soc Food Sci Nutr. 2000; 29:948–956.
21. Maskarinec G, Singh S, Meng L, Franke AA. Dietary soy intake and urinary isoflavone excretion among women from a multiethnic population. Cancer Epidemiol Biomarkers Prev. 1998; 7:613–619.
22. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.
23. Lee S, Park HS, Kim SM, Kwon HS, Kim DY, Kim DJ, Cho GJ, Han JH, Kim SR, Park CY, Oh SJ, Lee CB, Kim KS, Oh SW, Kim YS, Choi WH, Yoo HJ. Cut-off points of waist circumference for defining abdominal obesity in the Korean population. Korean J Obes. 2006; 15:1–9.
24. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F. American Heart Association. National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–2752.
25. Wu X, Cai H, Gao YT, Dai Q, Li H, Cai Q, Yang G, Franke AA, Zheng W, Shu XO. Correlations of urinary phytoestrogen excretion with lifestyle factors and dietary intakes among middle-aged and elderly Chinese women. Int J Mol Epidemiol Genet. 2012; 3:18–29.
26. Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S. JPHC Study Group. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007; 116:2553–2562.

27. de Kleijn MJ, van der Schouw YT, Wilson PW, Grobbee DE, Jacques PF. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S.women: the Framingham study. J Nutr. 2002; 132:276–282.

28. Nikander E, Tiitinen A, Laitinen K, Tikkanen M, Ylikorkala O. Effects of isolated isoflavonoids on lipids, lipoproteins, insulin sensitivity, and ghrelin in postmenopausal women. J Clin Endocrinol Metab. 2004; 89:3567–3572.

29. Gallagher JC, Satpathy R, Rafferty K, Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause. 2004; 11:290–298.

30. Willett W. Nutritional epidemiology. 2nd ed. New York: Oxford University Press;1998.
31. Ward H, Chapelais G, Kuhnle GG, Luben R, Khaw KT, Bingham S. European Prospective into Cancer-Norfolk cohort. Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European Prospective into Cancer-Norfolk cohort study. Breast Cancer Res. 2008; 10:R32.

32. Murkies A, Dalais FS, Briganti EM, Burger HG, Healy DL, Wahlqvist ML, Davis SR. Phytoestrogens and breast cancer in postmenopausal women: a case control study. Menopause. 2000; 7:289–296.

33. den Tonkelaar I, Keinan-Boker L, Veer PV, Arts CJ, Adlercreutz H, Thijssen JH, Peeters PH. Urinary phytoestrogens and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2001; 10:223–228.
34. Liu B, Qin L, Liu A, Uchiyama S, Ueno T, Li X, Wang P. Prevalence of the equol-producer phenotype and its relationship with dietary isoflavone and serum lipids in healthy Chinese adults. J Epidemiol. 2010; 20:377–384.

35. Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, Watanabe S. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000; 10:127–135.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download