Abstract
This study was performed to investigate the status of food restriction and the list of restricted foods in children with moderate to severe atopic dermatitis (AD), and to find out the effect of food restriction on the changes in nutrient intake and the severity of the disease. Sixty two patient children aged 12 months to 13 years presenting AD with a SCORing of Atopic Dermatitis (SCORAD) index between 20 and 50 were enrolled. The presence of food limitation, and list of restricted foods were surveyed through the caretakers and the patients were divided into 3 groups by the number of restricted food: non-restricted group, one to three restricted group, and more than three restricted group. Dietary intake was assessed for 3 months using a food frequency questionnaire (FFQ). Half of the subjects restricted foods. The restriction was higher in the order of soda, food additives, walnut, peanut, and other nuts as a single food item; and shellfish and crustacean group, processed foods, nuts, milk & dairy products, and meats as a food group. More than three restricted group ingested more fruits and less fish and meats, resulting in high consumption of vitamin C (p = 0.027). No significant difference in the ratio of nutrient intake by the number of restricted foods was observed in other nutrients. Significant improvement of AD symptom was observed in non-restricted group (p = 0.036) and one to three restricted group (p = 0.003). It is necessary to provide proper nutrition information and systematic and continuous nutrition management for balanced nutrient intake and disease improvement in children with AD.
Atopic dermatitis (AD) is one of the most common allergic diseases and manifests as a chronic recurrent dermatitis with itching. AD causes various physical problems due to frequent skin damage and itchy sensation, which decrease quality of life. In younger patients, the disease can be sufficiently serious as to disrupt friendships, learning performance, and family relationships, thus negatively influence the overall quality of life in addition to the physical problems [1].
Food allergy (FA) strongly influences AD [2]. Among patients with AD, 35-40% are accompanied by FA, which has been reported as 4 times higher in young children compared to adults [3]. The most effective way of treating FA is to restrict the diet to prevent the intake of allergy causing foods [4]. Skin symptoms can be markedly improved through the dietary restriction of allergy-inducing foods that cause AD [5]. However, dietary restriction should not be cavalier, since not all foods induce AD symptoms [6]. A restricted diet continued unnecessarily due to incorrect diagnosis [7] can delay the growth of atopic children and cause nutritional deficiency. Therefore, a correct diagnosis by professionals is crucial when restricting foods, and must be accompanied by careful nutritional management considering alternative/complementary diets for restricted foods.
This study was performed to investigate the presence of food restriction and the list of restricted foods in children with moderate AD, and to find out the effect of restricted diet on the changes in nutrient intakes and the severity of the disease. It is hoped that this study would provide fundamental data for the preparation of dietary guidelines and nutritional counseling for children with AD.
The study was performed from July 2010 to December 2011. Patients aged 12 months to 13 years presenting with AD were enrolled. The diagnosis of AD was based on previously defined criteria [8]. The inclusion criteria were a SCORing of Atopic Dermatitis (SCORAD) index between 20 and 50, no other systemic illnesses, and no administration history of antihistamines or systemic corticosteroids during the past week.
General characteristics including sex, age, presence of food limitation, and list of restricted foods were surveyed through the caretakers of AD patients who visited the Allergy Center of C hospital. The list of restricted foods in the survey questionnaire included eggs (egg), milk & dairy products (milk, cheese, plain yogurt), beans (Doenjang, tofu, soy milk), nuts (peanut, walnut, other nuts), grains (wheat, buckwheat), shellfish & crustacean (shrimp, crab, shellfish, abalone, oyster, squid, small octopus, octopus), fish (white flesh fish, red flesh fish), meats (beef, chicken, pork), processed foods (soda, food additives), and others, which are common causes of FA related to AD.
The diagnosis of AD of the subjects was made by pediatricians according to the prior criteria [8], and the degree of symptoms was clearly judged by the SCORAD index. This severity grading is composed of objective and subjective scores. The objective score takes into account the extent and the severity of AD lesions, such as erythema, edema/population, oozing/crust, excoriation, lichenification, and dryness. The subjective score includes evaluation of pruritus and insomnia using a visual analogue scale ranging from 1 to 10 [9].
During the study period, parents were instructed to take a bath once daily with mild acidic soap and warm water and to continuously treat their child's AD lesions. After the standardizing the skin management, we observed the change of severity of AD for 4 months.
Dietary intake was surveyed for the frequency of food intake for a 3-month period using a food frequency questionnaire (FFQ) [10] developed and proven for nutrient intake survey by the Korean Genome and Epidemiology Study (KoGES). The list of foods consisted of a total of 74 items including cooked rice (3), noodles (3), breads (3), other grains (3), potatoes (2), meat (8), egg (1), dairy products (3), beans (4), nuts(1), fish & shellfish (8), Kimchi (2), vegetables (12), mushroom (2), seaweed (2), fruits (12), beverage (1), and snacks (4). The reference intake for one portion intake was decided considering the median value of 24 h recall method, and then divided into 'less' (0.5 times), 'normal', and 'more' (1.5 times) [11]; for some foods, the portion size was presented with pictures showing their actual size. The frequency was divided into nine levels including 'twice a day' to 'less than once a month'.
Nutrient intakes were analyzed on the basis of the FFQ using the KoGES nutrition survey tool (Genimic FFQ ver. 1.0) of the Korea Centers for Disease Control and Prevention.
Individual intake for 14 nutrients (energy, protein, calcium, phosphorus, iron, zinc, vitamin A, vitamin E, vitamin B1, vitamin B2, niacin, vitamin B6, folic acid, vitamin C) was calculated, and the per capita daily nutrient intake was calculated from the total of nutrients from each food list [12].
The percent ratio of energy and nutrients to the recommended intake (%KDRIs, calculated as (nutrient intake ÷ RNI or AI) × 100) of nutrient intake for KDRI [12] was comparatively analyzed using the Recommended Nutrient Intake (RNI) or Adequate Intake (AI) of the KDRIs.
Statistical analysis for collected data was performed using SPSS for Windows, version 18.0 (SPSS, Chicago, IL, USA). The survey results were expressed as frequency and percentage, and mean ± SD. Student's t-test and ANOVA (Duncan's multiple range test) were performed to compare the difference in the means among groups.
The general characteristics of the subjects are shown in Table 1. The 62 children included 33 boys (53.2%) and 29 girls (46.8%). The age distribution by sex using 2-year intervals showed that children between 5-7 years of age constituted the highest ratio (21.0%). In boys, the 5-7 year age group was the highest (24.2%). In girls, the 3-5 year age group was the highest (27.6%).
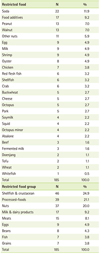
The number/types of restricted food groups in children with AD are listed in Tables 2 and 3. Thirty one (50.0%) patients had no food restriction. Restrictions of 1-3, 3-10, and over 10 foods were noted in 19.4%, 24.2%, and 6.4% of children, respectively. Among the restricted foods, soda (11.9%) was the highest, followed in decreasing order by food additives (9.2%), walnut (7.0%), peanut (7.0%), and other nuts (5.9%). Twenty seven of the restricted foods were classified into food groups including eggs, milk & dairy products (milk, cheese, plain yogurt), beans (Doenjang, tofu, soy milk), nuts (peanut, walnut, other nuts), grains (wheat, buckwheat), shellfish & crustacean (shrimp, crab, shellfish, abalone, oyster, squid, small octopus, octopus), fish (white flesh fish, red flesh fish), meats (beef, chicken, pork), and processed foods (soda, food additives). The restriction for the shellfish & crustacean was the most prevalent (24.9%), followed in decreasing order by processed foods (21.1), nuts (20.0%), milk & dairy products (9.2%), and meats (8.1%).
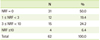
The %KDRIs by the number of restricted foods is shown in Table 4. The ratio of vitamin C intake for the recommended intake was significantly high in more than three restricted group (NRF ≥ 3; 228.5%) compared to non-restricted group (NRF = 0; 137.3%) and one to three restricted group (1 ≤ NRF < 3; 97.3%) (p < 0.05). There were no significant differences in the ratio of nutrient intake depending on the number of restricted foods.
Table 5 shows the ratio of food intake from each group by the number of restricted foods. The foods eaten by the subjects were divided into five food groups (grains, fish & meats vegetables, fruits and milk & dairy products). The intake (g) of each food group was divided by the intake (g) of all foods consumed to calculate the intake ratio (%). The intake ratio of each food group was compared among the non-restricted group (NRF = 0), one to three restricted group (1 ≤ NRF < 3) and more than three restricted group (NRF ≥ 3). Even though there was no significant difference, more than three restricted group ingested more fruits (19.4 ± 16.9) and less fish and poultry (7.4 ± 5.0) than the non-restricted group and one to three restricted group, suggesting that food consumption pattern resulted in high intake of vitamin C in more than three restricted group.
The SCORAD score of the subjects was measured for 4 months and the degree of improvement was evaluated using this score. Changes of SCORAD scores between the first and last month depending on the number of restricted foods are depicted in Figure 1. The average SCORAD score of the non-restricted group and one to three restricted group decreased significantly with time.
In this study, the effects of food restriction, types of restricted foods, and restricted diet on the changes in nutrient intakes and the severity of the disease were investigated in children aged 1-13 years with moderate to severe AD. Half of the subjects were restricted in some foods, with restrictions to up to three foods in 19.4%, 3-10 foods in 24.2%, and more than 10 foods in 6.4%. The subjects in this study, although they were patients with at least moderate AD, had fewer number of restricted foods compared to those in previous reports [13]. It might be due to the higher age in our subjects, suggesting the food restriction was eased off as the age of the subjects became older.
The restricted foods, in decreasing order of frequency, were soda, food additives, walnut, peanut and other nuts. When foods were grouped, the crustacean group was the most frequently restricted group, followed by processed foods, nuts, milk & dairy products, and meats. Various chemical ingredients, such as flavoring agents or preservatives in foods, misleadingly have been considered as main AD inducers [14], consistent with the predominance of dietary restriction of processed foods including soda and food additives in this study.
Restriction of the causative food(s) is very important in the treatment of FA, which is highly related to AD, since this will prevent the food allergens from entering in the body [15]. Because strict limitation on foods and their processed products is required in a restricted diet, proper education and training to distinguish processed foods containing food allergens is prudent. Several studies have reported improvements in symptoms after practicing a restricted diet in which problematic foods are strictly limited [16]. The intake of alternative foods that nutritionally complement the restricted foods should be arranged to prevent nutritional deficiency. Also, proper education is needed for young patients and their caretakers to plan a nutritionally balanced restricted diet.
Presently, calculation of %KDRIs according to the number of restricted foods showed that ratio of vitamin C intake was significantly high in more than three restricted group compared to the non-restricted group and one to three restricted group. Although it has been reported that the ratio of nutrient intake can be decreased by food restriction [17] and the ratio of insufficient nutrient intake can be high [13], and cases of nutritional deficiency due to insufficient intake of some nutrients have been observed [18], the present study did not show significant differences. This emphasizes the importance of nutrition management and the nutrient intake from alternative foods, as problems arise because of constant increase of children with AD and the restriction of foods [19]. Thus, it is necessary to continuously provide correct information and nutrition management for children with moderate to severe AD. Also, the ratio of vitamin C intake was significantly high in more than three restricted group, supporting the hypothesis that vitamin C helps improve chronic inflammation and positively influences AD [20].
Tanaka et al. [21] reported that the food preference of patients with AD showed lower preference to milk & dairy products, fish, and vegetables, which are known to induce allergy, while the preference for fruits was high and the food intake frequency of patients with AD was also lower in food groups such as fish, vegetables and nuts, which is similar to the result of food preference [22]. However, the ratio of food intake from each food group by the number of restricted foods was not significantly different in grains, fish & meats, vegetables, fruits and milk & dairy products among three groups. About 63.3% of patients with AD have accompanying FA [23]. In particular, in small children the development of AD is greatly influenced by food [24]. Sampson [5] reported that symptoms on the skin distinctively improved when the proper restricted diet was established after correct diagnosis of food allergens that induce AD.
Presently, changes in the severity of AD were investigated by comparing SCORAD scores between the second and fifth visits over 4 months. The SCORAD score was decreased by 4.9 (p < 0.05) and 10.15 (p < 0.01) in the non-restricted group and one to three restricted group, respectively, and improved with significant differences. However, no significant difference was observed in more than three restricted group, suggesting that food restriction is associated with the severity of AD without nutritional deficiency. Further studies on the association nutrition and severity of AD are needed.
The effects of food restriction on the changes in nutrient intakes and the severity of the disease in children with AD were investigated. Half of the subjects consumed a diet that was restricted. Significant improvement of AD symptom was observed in less restricted food group, suggesting that food restriction is associated with the severity of AD. It is necessary to provide proper nutrition information and systematic and continuous nutrition management for balanced nutrient intake and disease improvement in children with AD.
Figures and Tables
Figure 1
Comparison of the change in SCORAD index by the number of restricted foods. Significantly different by t-test. *p < 0.05, †p < 0.01. SCORAD index: SCORing of atopic dermatitis index. NRF: number of restricted foods.

References
1. Park CK, Park CW, Lee CH. Quality of life and the family impact of atopic dermatitis in children. Korean J of Dermatology. 2007; 45:429–438.
2. Ahn SH, Seo WH, Kim SJ, Hwang SJ, Park HY, Han YS, Chung SJ, Lee HC, Ahn KM, Lee SI. Risk factors of moderate to severe atopic dermatitis in the first 6 months of life. Pediatr Allergy Respir Dis. 2005; 15:242–249.
3. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis: pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999; 104:S114–S122.

4. Fiocchi A, Martelli A. Dietary management of food allergy. Pediatr Ann. 2006; 35:755–756. 758–763.

5. Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999; 103:717–728.

6. Arshad SH. Food allergen avoidance in primary prevention of food allergy. Allergy. 2001; 56:Suppl 67. 113–116.

7. Chung SJ, Han YS, Chung SW, Ahn KM, Park HY, Lee SI, Cho YY, Choi HM. Marasmus and kwashiorkor by nutritional ignorance related to vegetarian diet and infants with atopic dermatitis in South Korea. Korean J Nutr. 2004; 37:540–549.
8. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.
9. Oranje AP, Stalder JF, Taïeb A, Tasset C, de Longueville M. Scoring of atopic dermatitis by SCORAD using a training atlas by investigators from different disciplines. ETAC study group. Early treatment of the atopic child. Pediatr Allergy Immunol. 1997; 8:28–34.

10. Ahn Y, Park YJ, Park SJ, Min H, Kwak HK, Oh KS, Park C. Dietary patterns and prevalence odds ratio in middle-aged adults of rural and mid-size city in Korean Genome Epidemiology Study. Korean J Nutr. 2007; 40:259–269.
11. Ahn Y, Lee JE, Paik HY, Lee HK, Jo I, Kim K. Development of a semi-quantitative food frequency questionnaire based on dietary data from the Korea National Health and Nutrition Examination Survey. Nutr Sci. 2003; 6:173–184.
12. The Korean Nutrition Society. Dietary reference intakes for Koreans. Seoul: The Korean Nutrition Society;2005.
13. Park SJ, Lee JS, Ahn K, Chung SJ. The comparison of growth and nutrient intakes in children with and without atopic dermatitis. Korean J Community Nutr. 2012; 17:271–279.

14. Korean Society of Lipidology and Atherosclerosis. Guidelines for treatment of hyperlipidemia. Seoul: Korean Society of Lipidology and Atherosclerosis;2003.
15. Kim YH. A study on the dietary treatments of atopic dermatitis. Thesis Collect Res Inst Korean Med. 2005; 14:1–14.
16. Yu J, Jeon GR, Lee KS, Lee SY. Effect of egg white elimination diet on clinical progress and specific IgE levels in egg white sensitized children with atopic dermatitis. Pediatr Allergy Respir Dis. 2004; 14:71–79.
17. Lee JS. Dietary intakes and growth in relation to severity of atopic dermatitis in children [MS thesis]. Seoul: Kookmin University;2009.
18. Campbell M, Lofters WS, Gibbs WN. Rastafarianism and the vegans syndrome. Br Med J (Clin Res Ed). 1982; 285:1617–1618.

19. Tay YK, Kong KH, Khoo L, Goh CL, Giam YC. The prevalence and descriptive epidemiology of atopic dermatitis in Singapore school children. Br J Dermatol. 2002; 146:101–106.

21. Tanaka T, Kouda K, Kotani M, Takeuchi A, Tabei T, Masamoto Y, Nakamura H, Takigawa M, Suemura M, Takeuchi H, Kouda M. Vegetarian diet ameliorates symptoms of atopic dermatitis through reduction of the number of peripheral eosinophils and of PGE2 synthesis by monocytes. J Physiol Anthropol Appl Human Sci. 2001; 20:353–361.

22. Chung YM, Kim BS, Kim NI, Lee EY, Choue R. Study of nutritional status, dietary patterns, and dietary quality of atopic dermatitis patients. Korean J Nutr. 2005; 38:419–431.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download