Abstract
This study was performed to identify dietary behavior such as snack consumption, night-eating and nutrients intake associated with gestational diabetes mellitus (GDM). The study was conducted on 219 normal glucose tolerance (NGT) subjects and 44 GDM subjects by using a questionnaire including dietary behavior, food frequency and 3-day food record. The mean age, OGTT, and delivery weight of GDM subjects were statistically higher than those in NGT. A larger proportion of NGT subjects consumed black coffee (49.8%) while the majority of GDM subjects (61.4%) drank mixed coffee with sugar and cream. Dairy products were the most frequently consumed snack item in NGT subjects (40.7%), while fruits were most frequently consumed food item in GDM subjects (34.4%). Many of NGT subjects (49.8%) answered that they hardly took night-eating snacks whereas most of GDM subjects (61.4%) took night-eating snacks more than once a week. For change of taste preference, the proportion of NGT subjects who showed less preference for salty taste (33.3%) or greasy taste (16.9%) was higher than that of GDM subjects (11.4%). Nutrient intakes of energy, fat, cholesterol, saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), carbohydrate, vitamin B1, vitamin B2, vitamin C, and vitamin E in GDM group were significantly higher than those in NGT group. Nutrient densities of SFA and vitamin C in GDM group were higher and nutrient density of calcium was lower than those in NGT group. Taken together, it is recommended to reduce night-eating snack and choose less salty and fatty foods, black-coffee rather than coffee with cream and sugar, and more dairy products to prevent GDM.
Gestational diabetes mellitus (GDM) is defined as a state of glucose intolerance developed during pregnancy [1]. The prevalence of GDM is approximately 2% to 3% of Koreans, which is similar to that of other countries [2]. However, according to updated diagnostic criteria, the prevalence of GDM reaches 4% of Koreans [3]. GDM is a common complication during pregnancy, and is associated with maternal, prenatal morbidities, and higher incidence of diabetes afterward [4]. GDM can cause preeclampsia, hypertension, and caesarean section on mother and macrosomia, birth trauma, hypoglycemia, jaundice, hypocalcaemia, and hyperbillirubinemia on fetus [4]. Furthermore, GDM may increase the risk for future glucose intolerance and recurrence in the next pregnancy [5]. GDM uncovers a preexisting metabolic abnormality and return to normal glucose level after delivery, but may precede the development of overt diabetes mellitus later in life [6].
Major risk factors for GDM are older age in pregnancy, race or ethnicity, a family history of diabetes, physical activity, excess adiposity, and diet [7,8]. Appropriate nutritional management during GDM to maintain blood glucose within normal ranges throughout pregnancy can improve maternal, fetal, and neonatal adverse outcomes [9]. Nutrients associated with GDM risk include refined carbohydrates, animal fat, and heme iron [8,10]. Balanced three meals and snack consumptions are recommended to maintain normal body weight and to prevent ketone body production during pregnancy. Most of the diabetes mellitus patients consumed more snack than normal [11]. Therefore, inappropriate snack consumption may increase the risk of GDM. In addition, fruits provide vitamin C that increases iron absorption and prevent iron deficient anemia which happens frequently during pregnancy [12].
Night-eating is defined as eating at late time, a light meal as a meal substitution or food intake [13]. Frequent night-eating can cause a high calorie intake and because of this, side effects can be increased, including nutritional imbalance and excess intake of the sodium, obesity, and gastrointestinal disorder [14]. Stunkard [15] has reported that there was more food consumption in the subjects who had night-eating snack than the subjects who did not. Furthermore, night-eating subjects gained 5.2 kg of body weight compared to 0.9 kg body weight gain in non-night-eating subjects 6 years later [16]. Most night-eating snacks contain high concentration of fat and carbohydrate, which can increase calorie and fat intakes. It has been reported that intake of high carbohydrate may increase the risk of GDM [17]. And pre-pregnancy and pregnancy adherence to healthful dietary habit may be associated with lower risk of GDM [17,18]. There are very few studies observing dietary behaviors, including snack consumptions and particularly night-eating snacks in GDM. Since GDM increases the progression of type 2 diabetes mellitus and cardiovascular disease after delivery, it is important to control dietary behavior, including snack consumption for the prevention and treatment of GDM.
This study was performed to identify characteristics of dietary behavior, including snack intake and nutrient intakes in GDM and normal glucose tolerance (NGT) subjects and to evaluate their association with the blood glucose in pregnant women, which contribute to the prevention of GDM and its complications.
The study subjects were pregnant women who visited an endocrine clinic of a general hospital in Seoul, Korea from January, 2009 to December, 2010. The pregnant women who did not agree to the terms of the study and those who were diagnosed of diabetes mellitus or GDM previously, and those who were pregnant twins were excluded. A total of 287 pregnant women who diagnosed of GDM were surveyed. To diagnose GDM, the modified Carpenter & Coustan test consisted of the two step procedures, including screening test and diagnostic test were performed as described previously [19]. Briefly, the standard 50 g oral glucose tolerance test (OGTT) was performed after an overnight fast at 24-28th weeks of gestation in all pregnant women. The pregnant women with value of 140 mg/dL of plasma glucose or more of plasma glucose after 1 hr 50 g OGTT was subjected to additional diagnostic test consisted of 100 g OGTT. The GDM was diagnosed based on at least two abnormal plasma glucose values among fasting value of 95 mg/dL, 1 hr value of 180 mg/dL, 2 hr value of 155 mg/dL, or 3 hr value of ≥ 140 mg/dL on a 100 g OGTT. After the two step procedures, 263 participants were classified into two groups: (1) NGT group (n = 219) and (2) GDM group (n = 44) after excluding subjects who withdrew from the study (n = 22) and who transferred to other hospital (n = 2). This study was approved by the Institutional Review Board (IRB No. CGH-IRB-2006-22), and all participants provided written consent.
The general characteristics, including age, pre-pregnancy body weight, education, income, parity, and abortion experience were surveyed using a questionnaire. The height (cm) and body weight gain (kg) during pregnancy were obtained on the diagnostic test day by direct measurement. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared (kg/m2). The body weight at delivery (kg), delivery time (week), baby weight (g), and the sex of baby were collected from the delivery record. Blood was collected from subjects by clinical nurses. Fasting plasma glucose, 50 g and 100 g OGTT, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured.
Dietary behavior was investigated using a questionnaire. Dietary behaviors before and after pregnancy, including frequency of coffee drinking, snack, and night-eating habit. Furthermore, taste preference, including sweet, salty, spicy, sour, and greasy were surveyed by certified clinical dietitian. To assess nutrient intake, 3-day food record (2 week days and 1 weekend day) was obtained. The CAN Pro 3.0 (Computer Aided Nutritional Analysis Program, version 3.0, Korean Nutrition Society, Seoul, Korea) was used to analyze nutrient intake of the subjects.
The SPSS program (for windows version 12.0) was used to analyze data. Data were described as frequency, percentage, or mean ± standard deviation. The difference between two groups (NGT, GDM) was compared and student t-test was used to analyze the statistical significance. In order to compare the dietary behaviors of subjects, Chi-square test was used for the differences in frequency. Pearson's correlation coefficient analysis was used to determine the correlation between fasting plasma glucose and major nutrients intakes. Statistical significances were verified at the level of p < 0.05.
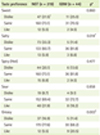
The general characteristics of the subjects are shown in Table 1. Among 263 pregnant women participated in this study, the number of NGT subjects was 219 and that of GDM subjects was 44. The mean age of GDM group (35.2 ± 3.5 years) was significantly higher than that of NGT group (33.8 ± 3.7 years). However, other variables, including education, occupation, income, parity, and abortion experience, did not show any differences between two groups.
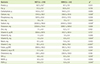
The clinical characteristics of the subjects are described in Table 2. Pre-pregnant BMI as well as pregnant BMI was not significantly different between two groups. Mean values of SBP and DBP in GDM group were significantly higher than those in NGT group (SBP: 115.2 ± 13.4 mmHg vs. 106.6 ± 11.8 mmHg, p < 0.05; DBP: 66.0 ± 15.8 mmHg vs. 61.1 ± 7.9 mmHg, p < 0.001). The mean values of both 50 g OGTT and 100 g OGTT were significantly higher in GDM subjects than those in NGT subjects. Particularly, 100 g OGTT after 1 hr (p < 0.008), 2 hr (p < 0.001), and 3 hr (p < 0.001) was significantly higher in GDM group than that of NGT group. Furthermore, delivery weight of GDM subjects was significantly higher than that of NGT subjects (67.3 ± 8.2 kg vs. 66.0 ± 15.8 kg, p < 0.05), whereas other factors, including delivery time (week), baby weight, and sex of baby were not significantly different between two groups.
The results for the frequency and types of coffee, snack consumption and night-eating habits in NGT subjects and GDM subjects during pre-pregnancy and pregnancy are presented in Table 3. The frequencies and types of coffee were not significantly different between two groups during prepregnancy, however, both frequencies and types of coffee were different during pregnancy. Although most of the subjects responded 'rarely' for frequency of the coffee consumption in both groups during pregnancy, more of GDM subjects answered 'rarely' compared to NGT subjects during pregnancy (p < 0.039, 70.5% vs. 49.8%). In particular, while 61.6% of pregnant women in NGT group drank black coffee, most of the pregnant women in GDM (61.4%) drank coffee mix that contained sugar and cream (p < 0.001).
Frequency of snack intake during pre-pregnancy period was not significantly different during pregnancy period. The types of snack were not significantly different between two groups before pregnancy, however, two groups showed a difference during pregnancy (p < 0.008). Dairy products were the most frequently consumed snack item (40.7%) in NGT subjects whereas fruits were the most frequently consumed snack item in GDM subjects (34.4%). Frequency and menu of night-eating were not significantly different between the two groups before pregnancy, however, they were significantly different during pregnancy (p < 0.015, p < 0.025). Most of NGT subjects (49.8%) answered that they rarely consumed night-eating snack, whereas most of GDM subjects (61.4%) took night-eating snack more than once a week during pregnancy. Furthermore, 'noodle' was the most frequently consumed night-eating snacks item in both groups (p < 0.025) during pregnancy.
The changes in the taste preferences during pregnancy are shown in Table 4. Among five tastes, preferences for sweet, spicy, and sour foods were not significantly different. However, the proportion of NGT subjects who showed less preference for salty taste was higher than that of GDM subjects (33.3% vs. 11.4%). For greasy taste, only 5.5% of the subjects answered "like" in NGT group, whereas 20.5% of the subjects answered "like" in GDM group.
Daily nutrient intake of the subjects is shown in Table 5. The average daily energy intake was 1973.9 ± 421.8 kcal for NGT group and 2127.3 ± 560.6 kcal for GDM group with a significant difference. Furthermore, the significant differences between two groups were observed in intakes of fat, carbohydrate, sodium, vitamin B2, vitamin C, vitamin E, cholesterol, saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and polyunsaturated fatty acid (PUFA). These nutrients intakes were higher in GDM subjects than those in NGT subjects.
Nutrient density was defined as nutrient intakes per 1000 kcal and nutrient densities were presented in Table 6. Although the mean daily intake of calcium was not significantly different between two groups, NGT group consumed 374 mg calcium per 1,000 kcal, which was significantly higher than that of the GDM group (338.0 ± 101.6 mg). Mean daily sodium intake was significantly higher in GDM group (5081.5 ± 1632.7 mg) than that in NGT group (4132.8 ± 1232.6 mg), however, sodium intake per 1,000 kcal was significantly higher in NGT group (2638 ± 786.8 mg) than that of GDM group (2434 ± 720.9 mg). The mean intakes of fat, vitamin E, cholesterol, and MUFA were higher in GDM group than those of NGT group, however, intakes of these nutrients per 1,000 were not significantly different between two groups. The mean intake of vitamin B2 was significantly higher in GDM (1.6 ± 0.6 mg) than it was in NGT (1.4 ± 0.5 mg), but nutrient density of vitamin B2 was significantly higher in NGT subjects (1.0 ± 0.4 mg) than it was in GDM subjects (0.9 ± 0.3 mg). The nutrient densities of vitamin C and SFA were consistently higher in GDM group than those were in NGT group.
The correlations between biochemical blood glucose markers, including fasting plasma glucose and nutrient intakes are shown in Table 7. Fasting plasma glucose was positively and significantly correlated with energy intake, and fat intake (p < 0.05). However, it was not significantly correlated with carbohydrate and protein.
In the present study, the snack consumption, night-eating, and nutrient intakes of 219 NGT subjects and 44 GDM patients who visited an endocrine clinic at a general hospital in Seoul were analyzed to investigate the association between the development of GDM and the mothers' dietary behavior and nutrient intakes.
The mean age of GDM group was statistically higher than that of NGT, the result of which was consistent with the previous study that reported age was an important risk factor of GDM [20]. It has been previously reported that fetal chromosomal abnormality, frequency of cesarean section, and the risk of premature amnion rupture among various prenatal complications are increased by aging [21].
OGTT of GDM patients was statistically higher than that of NGT subjects as reported in previous studies [22-24]. The diagnosis of GDM is typically based on the results of OGTT and there was a significant association between adverse maternal and prenatal outcomes and increasing fasting and 1 h and 2 h OGTT glucose values [25]. It has been reported that there was significant association between increasing maternal blood glucose and 1 h and 2 h OGTT glucose values with adverse pregnancy outcomes, including preterm delivery, gestational hypertension, and large-for-gestational-age infant [25,26]. These results of previous studies were consistent in our present study. It was previously reported that self management program such as improvement of eating pattern encouraged by clinical dietitians had positive effects on controlling glucose in diabetes mellitus patients [27]. GDM's delivery weight was significantly higher than that of NGT, which was consistent with several previous studies which reported that weight gain during pregnancy is associated with various prenatal complications and fetal growth retardation [28,29].
Frequency and types of coffee were significantly different between the two groups during pregnancy. Most of NGT subjects more consumed black coffee whereas the majority of GDM subjects drank instant coffee mix containing both sugar and cream. Since the coffee mix contains food additives and cream that is mainly composed of fat, drinking instant coffee mix may have adverse effects on the GDM mothers' health. Although 88.2% of Korean pregnant women replied that they continued to drink coffee in spite of their pregnancy in a previous survey, several studies reported that caffeine intake (above 300 mg per day) can increase the risk of newborn mortality [30]. It can be suggested that the Korean mothers nowadays are well aware of the negative aspects of coffee intake as shown by the reduced frequencies of coffee intake of both NGT and GDM mothers in the present study.
There was a difference between the NGT subjects and GDM subjects during their pregnancy regarding the type of snack menu. Dairy products were the most frequently consumed snack among NGT subjects, whereas the GDM group reported to have eaten fruits. Such difference may have affected nutrient density of calcium between NGT and GDM in this study. Nutrient density of calcium in NGT subjects was statistically higher than that of GDM in spite of no significant differences in terms of daily calcium intake. Several recent studies have reported that calcium intake is related to glucose level. Choi et al. [31] reported that high calcium intake was correlated with lower HbA1c levels, and Villegas et al. [32] showed that calcium intake had negative correlation with type 2 diabetes mellitus. It can be suggested that higher calcium intake of NGT compared to GDM in this study may not affect insulin secretion itself but it may have attenuated insulin resistance, thus having positively affected the NGT subjects' health status by lowering the risk of GDM. In contrast to NGT, the majority of GDM in the present study replied that they ate fruits for snack. Both daily intakes and nutrient density of vitamin C in NGT subjects were significantly higher than those in GDM. However, there is limitation to investigate the correlation between the amount of fruit intake was surveyed and GDM occurrence since the frequency of fruit intake was surveyed but the total amount was not. Furthermore, the sugar content may vary because of it highly depends on the kinds of fruit which the subjects had taken. Therefore, further investigations are needed taking into account kinds and amount of fruit consumed.
Frequency and type of night-eating menu were also significantly different between the NGT and GDM subjects. There were no significant differences of frequency and type of night-eating menu between the two groups during prepregnancy. For frequency of night-eating consumption, most of NGT subjects answered "rarely" whereas most of GDM subjects consumed night-eating snacks more than once a week during pregnancy. Due to excessive night-eating snack, high-calorie consumption can be a causative factor for the obesity and gastrointestinal disorder [14]. In case of NGT group, the lower frequency of night-eating snack from 'more than once a week' to 'rarely' may decrease the risk of GDM.
Despite of its inherent limitations of reflecting only subjective opinions, taste preference investigations showed that the proportion of GDM subjects who answered "same" and "like" was much higher than that of NGT subjects and mean daily sodium intake was significantly higher in GDM group than in NGT group, which was consistent with the previous study [33]. It has been reported that salty foods are consumed significantly more in the third trimester and this may be associated with a higher threshold for saltiness. Pregnant women in the third trimester rated less salty than other stages of pregnancy. This salty taste alteration may change salt consumption and cause fluid retention and hypertension [34]. In fact, the values of SBP and DBP in GDM group were significantly higher than those in NGT group. It has been reported that there is association between GDM and gestational hypertension [35]. The rates of both gestational hypertension is increased in women with GDM [36,37] and the rate of preeclampsia, a category of gestational hypertension, is affected by the severity of GDM [38]. Hypertension in GDM was associated with physiologic abnormal characteristics, including insulin resistance and over expression of innate immune response, which is related with inflammation and oxidative stress, and vascular abnormality [35].
Furthermore, more GDM subjects replied that they prefer 'greasy' taste compared to NGT subjects during pregnancy. This taste preference difference may affect the mean daily intakes of fat, cholesterol, and SFA in GDM which were significantly higher than those in NGT group. It has been reported that cholesterol intake was positively correlated with the risk of stress and mental stress during pregnancy and can affect the fetus in negative ways such as fetal growth retardation and low fetal weight [39]. High levels of cholesterol during pregnancy can change fetal aorta and increase the susceptibility to cardiac disorders such as coronary arteriosclerosis or fat formation [40]. In the present study, a positive correlation between fat intake and the level of blood fasting glucose was observed. Therefore, reducing fat intake may help GDM patients to prevent complications of GDM and reduce negative effects on their fetus.
The strength of our study lies in the fact that we investigated dietary behaviors of not only daily snack, but also night-eating snack in GDM. However, this study has limitations, including small number of GDM subjects and usage of FFQ that is not semi-quantified. Further studies incorporating large number of subjects and more accurate measurement of intake amounts may be able to analyze the correlations between the dietary behavior and the occurrence of GDM as well as its relative risk.
In conclusion, the present study reported that the significant differences in snack intake, night-eating habits, and nutrient intakes between NGT and GDM subjects. We recommend healthy snacks and appropriate dietary behaviors as two important lifestyle factors that can help reduce the risk of GDM and its complications. Particularly, it would be beneficial to consume plenty of vegetables, reduce night-eating snacks consumption, choose less salty and fatty foods, drink black-coffee rather than instant coffee mix with cream and sugar, and consume dairy products to prevent GDM.
Figures and Tables
Table 2
Biochemical analysis of the subjects

NGT: normal glucose tolerance subjects, GDM: gestational diabetes mellitus subjects, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, OGTT: oral glucose tolerance test.
*Mean ± SD; †Analysis by Student t-test; ‡p < 0.05; §p < 0.01; ∥p < 0.001; ¶Analysis by Chi-square.
Acknowledgements
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (Grant No. A102065-1011-1070100).
References
1. Metzger BE, Coustan DR. The Organizing Committee. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 1998; 21:Suppl 2. B161–B167.
2. Jang HC, Jung KB, Cho NH, Metzger BE. Gestational diabetes mellitus in Korea: is universal screening necessary? Korean J Obstet Gynecol. 1996; 39:519–530.
3. Jang HC, Cho YM, Park KS, Kim SY, Lee HK, Kim MY, Yang JH, Shin SM. Pregnancy outcome in Korean women with gestational diabetes mellitus diagnosed by the Carpenter-Coustan criteria. J Korean Diabetes Assoc. 2004; 28:122–130.
5. Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care. 2007; 30:1314–1319.
6. Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001; 86:989–993.

7. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004; 27:Suppl 1. S88–S90.
8. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011; 94:1975S–1979S.

9. Jovanovic L. Medical nutritional therapy in pregnant women with pregestational diabetes mellitus. J Matern Fetal Med. 2000; 9:21–28.

10. Anh YJ, Paik HY, Lee HK, Park YS. Comparison of food intakes between newly diagnosed diabetics and nondiabetics by food frequency questionnaire in adults living in rural area of Korea. J Korean Soc Food Sci Nutr. 1998; 27:182–190.
11. Yang EJ, Kim WY. The influence of dietary factors on the incidence of non-insulin-dependent diabetes mellitus. Korean J Nutr. 1999; 32:407–418.
13. Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome; a pattern of food intake among certain obese patients. Am J Med. 1955; 19:78–86.
14. Kim MH, Jeong ES, Kim EJ, Cho HK, Bae YJ, Choi MK. Night eating status of university students in partial area of Chungnam. J East Asian Soc Diet Life. 2011; 21:563–576.
15. Stunkard A. Two eating disorders: binge eating disorder and the night eating syndrome. Appetite. 2000; 34:333–334.

16. Andersen GS, Stunkard AJ, Sørensen TI, Petersen L, Heitmann BL. Night eating and weight change in middle-aged men and women. Int J Obes Relat Metab Disord. 2004; 28:1338–1343.

17. Ji SK, Jang HC, Choi H. A case-control study of food habits and diet intakes of women with gestational diabetes mellitus. Korean J Nutr. 2008; 41:41–53.
18. Tobias DK, Zhang C, Chavarro J, Bowers K, Rich-Edwards J, Rosner B, Mozaffarian D, Hu FB. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr. 2012; 96:289–295.

19. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982; 144:768–773.

20. Di Cianni G, Volpe L, Lencioni C, Miccoli R, Cuccuru I, Ghio A, Chatzianagnostou K, Bottone P, Teti G, Del Prato S, Benzi L. Prevalence and risk factors for gestational diabetes assessed by universal screening. Diabetes Res Clin Pract. 2003; 62:131–137.

21. Park HJ, Lee SH, Cha DH, Kim IH, Jun HS, Lee KJ, Song SA, Park HR, Chung CJ, Lee CN. Pregnancy outcomes in women aged 35 and older. Korean J Obstet Gynecol. 2006; 49:2066–2074.
22. Jeoun HJ, Kim HH, Kim SY, Sung IK, Lee WB, Chun CS, Lee GS, Kim SJ. Appropriate timing of the screening test of gestational diabetes. Korean J Perinatol. 2006; 17:217–224.
23. Yi KW, Jung JW, Shin JH, Oh MJ, Lee JK, Hur JY, Saw HS, Park YK. Perinatal outcomes in pregnant women with impaired glucose tolerance (IGT) proven through 100 g oral glucose tolerance test (OGTT). Korean J Perinatol. 2006; 17:25–32.
24. Hwang YJ, Park BK, Park S, Kim SH. A comparative study of eating habits and food Intake in women with gestational diabetes according to early postpartum glucose tolerance status. Diabetes Metab J. 2011; 35:354–363.

25. Black MH, Sacks DA, Xiang AH, Lawrence JM. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care. 2010; 33:2524–2530.

26. HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008; 358:1991–2002.

27. Park JY. A study on self management, hemoglobin A1c (HbA1c), and perceived health status for the type II diabetes patients. J Korean Biol Nurs Sci. 2010; 12:106–113.
28. Cho AR, Kyeung KS, Park MA, Lee YM, Jeong EH. Risk factors of gestational diabetes mellitus. Korean J Perinatol. 2007; 18:329–337.
29. Yoo YW, Ha JY, Kang CS, Park SC, Park JK. A study of the factors associated with the pattern of gestational weight gain. Korean J Obstet Gynecol. 2010; 53:23–28.

30. Oh SM, Chung KH. Coffee consumption during pregnancy in Korean and effect upon serum lipids level in maternal and umbilical cord blood. Yakhak Hoeji. 1998; 42:459–466.
31. Choi YM, Lee JH, Han JS. Effects of vitamin D and calcium intervention on the improvement of resistance in patients with type 2 diabetes mellitus. Korean Diabetes J. 2009; 33:324–334.

32. Villegas R, Gao YT, Dai Q, Yang G, Cai H, Li H, Zheng W, Shu XO. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: the Shanghai Women’s Health Study. Am J Clin Nutr. 2009; 89:1059–1067.

33. Faas MM, Melgert BN, de Vos P. A brief review on how pregnancy and sex hormones interfere with taste and food intake. Chemosens Percept. 2010; 3:51–56.

34. Bowen DJ. Taste and food preference changes across the course of pregnancy. Appetite. 1992; 19:233–242.

35. Sibai BM, Ross MG. Hypertension in gestational diabetes mellitus: pathophysiology and long-term consequences. J Matern Fetal Neonatal Med. 2010; 23:229–233.

36. Svare JA, Hansen BB, Mølsted-Pedersen L. Perinatal complications in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2001; 80:899–904.

37. Stella CL, O'Brien JM, Forrester KJ, Barton JR, Istwan N, Rhea D, Sibai BM. The coexistence of gestational hypertension and diabetes: influence on pregnancy outcome. Am J Perinatol. 2008; 25:325–329.

38. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004; 191:1655–1660.

39. Kim YJ, Lee SS. The relation of maternal stress with nutrients intake and pregnancy outcome in pregnant women. Korean J Nutr. 2008; 41:776–785.
40. Bae JG, Park JC, Rhee JH, Kim JI. Comparison of plasma lipid and lipoprotein concentrations in normal and intrauterine growth restriction pregnancies. Korean J Obstet Gynecol. 2009; 52:400–406.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download