Abstract
Understanding the relationship between energy nutrients compositions in a diet and appetite-controlling substances is essential for providing sound advice to anyone attempting to control body weight. Appetite is known to be affected by various hormones, ghrelin and peptide tyrosine-tyrosine (PYY), which are related to the compositions of a diet. The purpose of this study was to investigate the effects of compositions of energy nutrients in the diet on the levels of postprandial appetite-related hormones and satiety in healthy adult women. Ten subjects (BMI: 18.5-22.9 kg/m2) were recruited and assigned to three iso-coloric breakfast meals with different compositions of energy nutrients, regular meal (RM, CHO: 60%, Pro: 20%, Fat: 20%), high protein meal (HPM, CHO: 30%, Pro: 50%, Fat: 20%), and high fat meal (HFM, CHO: 30%, Pro: 20%, Fat: 50%). Blood levels of ghrelin, PYY, insulin and leptin and satiety were assessed at baseline, 30, 60, 90, 120, and 180 min following the consumption of each meal. There was no significant difference in the fasting blood hormones among the subjects taking each meals at baseline. Blood levels of ghrelin and insulin changed significantly following the consumption of each meal (p<0.05) over time, however no significant difference was shown between experimental meals until 180 min. Blood levels of PYY and leptin were not changed following the ingestion of each meals. In conclusion, the composition of energy nutrients in a diet had no effect on the postprandial plasma levels of ghrelin, PYY, insulin and leptin as well as satiety in healthy adult women.
The appetite is the need to eat the foods and is the condition that causes the need for ingesting the foods regardless the hunger. The appetite is controlled by the signal of appetite-related substance that is synthesized at the center and periphery in human body [1,2]. It is known that ghrelin and peptide tyrosine-tyrosine (PYY) are the short-term controlling hormones of appetite and insulin and leptin are the long-term controlling hormones [3]. It was reported that ghrelin and PYY that are the short-term controlling hormones are influenced by a single meal [3-5]. Ghrelin is the hormone secreted from stomach and involved in food intake and homeostasis of energy, and increases the food intake by improving the appetite in human body [6]. The secretion of ghrelin begins to increase before meal and shows the tendency of decrease after passing 1 to 4 hours after meal [7,8]. While, PYY secreted from the peripheral part of gastrointestinal tract suppresses the appetite and reduces the fasting state, and contrary to ghrelin, it decreases during the fasting state and begins to increase after meal and maintain the level for several hours [9,10].
It is known that the secretion of grelin and PYY are influenced by the calory consumption and composition of energy nutrients, however, it was reported that a single high protein meal increases the fullness and decreases food intake by increasing the secretion of PYY while meals with either high carbohydrate or fat does not exert the same effect [10,11]. In healthy females, the high carbohydrate meal significantly suppressed ghrelin secretion compared to the high fat meal [12].
It is known that leptin and insulin are long-term appetite controlling hormones which do not suppress the appetite directly after meal, but insulin secretion is stimulated with high carbohydrate meal and controls the appetite indirectly by suppressing the secretion of ghrelin. It was reported that leptin does not show the significant change with a single meal, but plays a role in long-term appetite control [12].
Appetite-related hormones are involved in controlling food intake by being influenced by energy nutrients composition, and short-term and long-term signals interact each other. However, most of the studies used liquid type meals, not the meal consisted of solid foods, which may have different effects on the secretion of appetite controlling hormones Few studies have examined a single meal effect on appetite-controlling hormone secretion, especially in Koreans. In this study, we investigated the effects of a single meal with different ratios of carbohydrate, protein and fat on the secretion of appetite control hormones in healthy Korean females.
This study was carried out from March to November, 2010. The study was approved by the IRB commeetee of Kyung Hee University (KHU IRB 2010-001). The healthy females whose body mass index (BMI) are above 18.5 kg/m2 and less than 23 kg/m2 were targeted for this study. All subjects were the ones whose weight had not been changed for last 3 months and non-smokers. Individuals taking stomach-related medicines or received the gastric surgery recently or have medical or psychological disease were excluded. Anyone who were judged to have problem with dietary behavior with 2.5< of the abstaining eating scale in dietary behavior survey [13] were also excluded from the study. A total of 12 subjects were recruited and 10 subjects participated in this randomized cross-over study after two dropped out at the beginning of the study.
Study subjects were randomly divided into 3, 3, and 4 and allocated in one of the three experimental meal group which are regular meal (RM), high protein meal (HPM) and high fat meal (HFM) Experimental meals were provided after overnight fast, and no additional food or beverage was allowed with the meal. The participants were instructed to listen to light music or read books while the experiment was carried out.
Questionnaire was used to evaluate dietary habits including meal size, mealtime regularity, speed of eating, frequencies of overeating and eat-outs. Food records were collected to measure dietary intake and the CAN Pro Version 3.0 (Computer Aided Nutritional Analysis Program, The Korean Nutrition Society) was used to calculate nutrient intake. The calorie intake was compared with estimated average requirement (EAR), and protein, calcium, phosphorus, iron, zinc, vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin C and niacin were compared with recommended intake (RI). Dietary fiber, potassium and vitamin E were compared with adequate intake (AI).
The height (cm) and weight (kg) of the subjects were measured with minimum clothes worn and shoes and socks taken off. Body composition was measured using body component analyzer (Inbody 4.0, Biospace, Korea). Body mass index (BMI) was acquired with the value that the weight (kg) was divided by the square of the height (m). Ideal body weight (% IBW) was acquired by multiplying 100 by the value that current weight (kg) was divided by ideal body weight (kg).
Experimental meals were isocaloric diet with different ratios of carbohyrate, protein and fat. Caloric supply was calculated based on the basal energy expenditure (BEE) of each subject by using Harris-benedict formula and multiplying that by activity factor, 1.3 (mean value: 520 kcal). Composition ratio of energy nutrients of the meal were as follows: regular meal (RM, CHO : Pro : Fat = 60 : 20 : 20), high protein meal (HPM, CHO : Pro : Fat = 30 : 50 : 20) and high fat meal (HFM, CHO : Pro : Fat = 30 : 20 : 50). RM was composed with rice, soybean paste soup, steamed sweet potato, chicken breast meat, lettuce salad, soybean sauce dressing and kkakdugi. For HPM, the amount of chicken breast meat was increased, the amount of rice was reduced, and steamed sweet potato was excluded. For HFM, french dressing was used instead of soybean sauce while the amount of rice and salad was maintained as those of HPM. The standard recipe was used to prepare experimental meals. Study subjects were instructed to finish the experimental meal within 20 minutes without leftovers.
The clinical nurse collected 5 ml of blood at 0, 30, 60, 90, 120 and 180 minutes after the meal by inserting catheter to antecubital vein of the subject. The gathered blood was stored in cold storage of -20℃ before the analysis after separating blood serum using the centrifugal separator (4℃, 3,500 rpm, 15 min). The concentration of ghrelin, PYY, insulin and leptin that are the appetite-related hormones were analyzed with Bio-plex equipment and Bio-plex manager 4.1.1.456 software by using Millipore, MILLIPLEX™ MAP human gut hormone panel (Millipore, Billerica, MA). The program that was used for the analysis was Upstate Beadview program.
In order to determine the degree of subjective fullness after meal, visual analogue scale (VAS) was used [14]. The subjects were instructed to express the appetite status before and after the meal in order to describe the degree of fullness while collecting the blood. VAS was composed of the following questionnaires, "How hungry are you?", "How full are you?" and "What do you want to eat more?". The subjects were instructed to describe the degree of appetite by marking with a pen in the 10 cm long scale from "very much" to "not at all" at the both ends of the questionnaire.
The data of the experiments were analyzed by using Statistical Package for the Social Science (SPSS) version 18.0 and the results were presented with mean, standard deviation (SD) or standard error (SE). Regarding the value of change along time after ingesting the experimental meals, one way repeated measures ANOVA test and paired t-test were used. Regarding the differences among the three groups, the statistical significance was evaluated through Kruskal-Wallis test and p<0.05 was considered as statistically significant.
The average age of the subjects who participated in this study was 22.3 ± 3.2 years. The average height and weight were 161.8 ± 5.3 cm and 52.4 ± 4.7 kg, respectively and as the results of calculating %IBW and BMI (kg/m2), all were within the normal range.
The results of analyzing dietary habits were shown in Table 1. Meal frequency data indicated that, only 40% of the subjects have 3 meals a day. Regarding the regularity of meal, only 20% of the subjects answered that they have regular meals, Regarding the speed of eating, 70% answered that they spend about 20 minutes. Sixty percent of the subjects have a habit of overeating and 90% eat snack and eat out more than 3 times a week.
Nutrient intake data indicated that the average total calorie intake per day was 1,629.1 ± 417.0 kcal, and the ratio of carbohydrate, protein and fat was 53.3 : 15.4 : 31.4, showing the calorie percent from fat was high when comparing it with the adequate energy ratio recommended by Dietary Reference Intakes for Koreans, 2010.
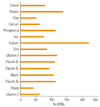
The results of comparing daily intake of nutrients to KDRIs is shown in Figure 1. The average calorie that the subjects consumed was 80% of KDRIs and protein was 139%, calcium was 63%, iron was 77%, and sodium was 222%. The intakes of vitamin A, B1, B2, niacin, vitamin B6 were close to the adequate intake reference, but the intakes of vitamin C (63%), folate (42%) and dietary fiber (52%) were very low.
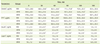
The results of measuring concentration of appetite-related hormones for 180 minutes. after consuming the experimental meals are shown in Table 2 and Figure 2, respectively. The concentration of ghrelin showed the significant reduction at 30 min (58.6 ± 25.6 pg/mL), 60 min (56.4 ± 26.6 pg/mL), 90 min (71.1 ± 72.8 pg/mL) and 120 min (78.9. ± 24.3 pg/mL) when compared to the value of baseline in RM (p < 0.05). HPM significantly reduced ghrelin at 120 min (52.6 ± 15.0 pg/mL) compared to the baseline (89.6 ± 26.1 pg/mL), HFM consumption resulted in the significant reduction at 60 min (61.2 ± 22.3 pg/mL), 90 min (72.5 ± 19.4 pg/mL), 120 min (78.6 ± 22.5 pg/mL) and 180 min (69.7 ± 25.2 pg/mL) compared to the value of baseline (p < 0.05). However, significance disappeared at 180 minutes.
The concentration of PPY did not show significant change for 180 minutes after experimental meals when compared to the value of the baseline. No difference was found in PPY concentration among experimental meals.
The concentration of insulin increased to the highest value at 30 minutes after all experimental meals and the concentration remained highest with RM compared to that of the other two meals. HPM increased insulin significantly only at 30 minutes (2.8 ± 0.7 ng/mL). No difference was found in insulin concentration between experimental meals.
The concentration of leptin did not show the significant change for 180 minutes after the consumption of experimental meals when compared to the value of baseline. No difference was found in insulin concentration between experimental meals.
The results of measuring visual analogue scale against hunger, fullness and the desire to eat in study subjects were shown in Figure 3. The hunger and desire to eat after consuming the experimental meals fell rapidly and showed the lowest level at 30 minutes, however the fullness showed the highest score at 30 minutes after the meal (p < 0.05). No difference was found in insulin concentration between experimental meals.
In order to investigate the effects of a single meal with different composition of energy nutrients on the appetite-related hormones, this study measured the concentration of appetite-related hormones after ingesting the meal of same calories with different ratio of energy nutrients, in the healthy female subjects.
When comparing daily energy intake of the subjects with the dietary reference intakes for Koreans [15], 80% of the EAR was consumed. It was similar the results form the National Health and Nutrition Examination Survey [16] which was 1,629.6 ± 35.9 kcal/day for adult females and 1,600.3 ± 535.4 kcal/day from the female college students in Seoul (18 kg/m2 ≤ BMI < 23 kg/m2) [17]. The protein intake was within the recommended range (7-20% of the calorie), however the fat intake of study subjects was 31.4% of the calorie which is above the recommend range (15-25% of the calorie). Study subjects revealed dietary habits of frequent snacking and eat-outs which may contributed to high fat consumption in this subjects. The experimental meal used in this study provided the 520 kcal which was around 31% of the average calorie consumption of the study subjects.
The appetite-controlling hormones influence food intake which in turn influences the secretion of appetite-controlling hormones. It is known that ghrelin decreases and PYY increases after meal, respectively. Results from this study indicated that the concentration of ghrelin was reduced after consuming the experimental meals, however no difference was found between meals. These results indicate that the composition ratio of energy nutrients does not influence on the secretion of ghrelin. In the studies of Smeets et al. [18] and Lejeune et al. [19], the concentrations of ghrelin were not influenced by the energy nutrient ratio.
Even though the ratio of energy nutrients did not affect the ghrelin concentration, the hours after meal could significantly affect the levels of ghrelin. In the study of Tannous et al. [20], the concentration of ghrelin was lowest at 60 minutes after HFM (50% of total calories) consumption. In this study, the concentration of ghrelin was also lowest at 60 minutes after HFM. On the contrary to our results, the studies of Erdmann et al. [21] and Monteleone et al. [22] indicated that, the level of ghrelin became the lowest at 180 minutes after ingesting liquid meal that contains 75% and 85% fat, respectively. The discrepancy could be explained by the fact that the different contents of fat included in the meals as well as the differences in the types of meal. Gastric outlet and generation of amino acid, etc. could be influencing factors.
While, PYY did not show any significant difference along with the ratio of energy nutrients in all experimental meals and the hours after the meal. A previous research [18] also showed that no significant difference in the secretion of PYY after ingestion of various experimental meals. Therefore, ratio of energy nutrients included in the meals did not influence sufficiently to induce the secretion of PYY for short period of time.
Insulin is secreted along with the intake of carbohydrate, but it is not clearly known that the secretion of insulin is influenced by the composition ratio of energy nutrients. In this study, there was no significant differences among the three experimental meals regarding the concentration of insulin although it is increased aftter the meal consumption. The contents of carbohydrate included in high protein and high fat meals were 39 g compared with 78 g in the regular meal. The study result of Blom et al. [12] also showed that there was no difference regarding the secretion of insulin after ingestion of experimental meals. According to what was suggested in the study of Schmid et al. [23], the meal with affluent protein stimulates the secretion of insulin as a result from the increased concentration of amino acid in the blood.
It has been reported that insulin as the long-term controlling factor like leptin, does not directly control the appetite however, it acts on the digested and absorbed glucose after meal by suppressing the concentration of ghrelin. [21,24,25]. Saad et al. [26] reported that the injection of insulin suppress the secretion of ghrelin by 25% while the injection was withdrawn the secretion of ghrelin bounced back to the basal level within 1 hour. In this study, insulin secretion increased while the concentration of ghrelin was reduced after meal. So, it can be speculated that the possibility of insulin as a indirect suppressor of appetite.
The concentration of leptin did not show any significant difference among the three experimental meals at the baseline. Leptin acted as a long-term appetite controller like insulin, however it did not act in short-term basis. [12,22]. Keim et al. [27] explained that in the balanced energy status, the appetite-controlling action of leptin was absent by a single meal, however, in case of imbalanced energy status, leptin suppress the appetite with a single meal.
As the results of measuring appetite after ingesting experimental meal, the degree of hunger and desire to eat increased significantly and the degree of fullness decreased after meals showing no significant difference along with the composition of energy nutrients. However, according to the studies of Monteleone et al. [22], the degree of hunger after ingesting high carbohydrate (77%) meal were more suppressed than in high fat (75%) meal.
This study is the one that investigate the secretion of appetite-related hormones along with the composition of energy nutrients targeting the healthy females. It is note worthy since the previous studies mainly used liquid meal mean while this study provided the experimental meals that are composed of solid food. As the limitation of the study, dietary habits of the subjects were not considered. It has been pointed out in the several studies [28,29] that the subjects' habitual meal type and meal time influence the secretion of appetite-related hormones. The other limitation is the concentrations of the hormones were in very wide range. It is proposed that consideration of the subjects' dietary habits and the large number of the subjects in future study.
A single meal with different ratio of energy nutrients did not influenced on the appetite-related hormones levels as well as the degree of hunger, fullness and desire to eat in health female adults. Meanwhile, the hours after one meal with different composition of energy nutrients significantly affected the levels of appetite hormones and the degree of hunger, fullness and desire to eat. In conclusion, appetite-related hormones might be influenced by many other factors including the types of meal, gastric outlet and generation of amino acid, etc. Further studies are needed to verify the effects of appetite influencing factors on the concentration of appetite-related hormones.
Figures and Tables
Figure 2
Percentage changes of (A) ghrelin, (B) PYY, (C) insulin, and (D) leptin for 3 hours after taking experimental meals. RM: regular meal, HPM: high protein meal, HFM: high fat meal. Values are mean ± SE. *Significantly different from baseline (0 min), as determined by paired t-test (p < 0.05).

Figure 3
Changes of (A) hunger, (B) fullness and (C) desire to eat for 3 hours after taking experimental meals. RM: regular meal, HPM: high protein meal, HFM: high fat meal. Values are mean SE. *Significantly different from baseline (0 min), as determined by paired t-test (p < 0.05).

References
1. Read N, French S, Cunningham K. The role of the gut in regulating food intake in man. Nutr Rev. 1994. 52:1–10.

3. Havel PJ. Peripheral signals conveying metabolic information to the brain: Short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med (Maywood). 2001. 226:963–977.

4. Blom WA, Lluch A, Stafleu A, Vinoy S, Holst JJ, Schaafsma G, Hendriks HF. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr. 2006. 83:211–220.

5. Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial PYY 3-36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab. 2008. 52:188–195.

6. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999. 402:656–660.

7. Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001. 24:RC19–RC21.

8. Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001. 86:5992–5995.

9. Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985. 89:1070–1077.

10. Pedersen-Bjergaard U, Høst U, Kelbaek H, Schifter S, Rehfeld JF, Faber J, Christensen NJ. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand J Clin Lab Invest. 1996. 56:497–503.

11. Anderson GH, Moore SE. Dietary proteins in the regulation of food intake and body weight in humans. J Nutr. 2004. 134:974–979.

12. Blom WA, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HF. Ghrelin response to carbohydrate-enriched breakfast is related to Insulin. Am J Clin Nutr. 2005. 81:367–375.

13. Van Strien T, Frijters J, van Staveren W, Defares P, Deurenberg P. The predictive validity of the Dutch Restrained Eating Scale. Int J Eat Disord. 1986. 5:747–755.

14. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000. 24:38–48.

15. The Korean Nutrition Society. Dietary reference intakes for Koreans. 2010. Seoul: Hanarum Publishers.
16. The Fourth National Health and Nutrition Examination Survey (KNHANES IV). 2008. Seoul: Korea Center for Disease Control and Prevention.
17. Lim JY, Na HB. Dietary macronutrients and VO2 by BMI among female college students in Seoul. Korean J Community Nutr. 2006. 11:52–62.
18. Smeets AJ, Soenen S, Luscombe-Marsh ND, Ueland Ø, Westerterp-Plantenga MS. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J Nutr. 2008. 138:698–702.

19. Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006. 83:89–94.

20. Tannous dit El Khoury D, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab. 2006. 50:260–269.

21. Erdmann J, Lippl F, Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul Pept. 2003. 116:101–107.

22. Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in heal thy women. J Clin Endocrinol Metab. 2003. 88:5510–5514.

23. Schmid R, Schulte-Frohlinde E, Schusdziarra V, Neubauer J, Stegmann M, Maier V, Classen M. Contribution of postprandial amino acid levels to stimulation of insulin, glucagon, and pancreatic polypeptide in humans. Pancreas. 1992. 7:698–704.

24. Greenman Y, Golani N, Gilad S, Yaron M, Limor R, Stern N. Ghrelin secretion is modulated in a nutrient- and gender-specific manner. Clin Endocrinol (Oxf). 2004. 60:382–388.

25. Erdmann J, Töpsch R, Lippl F, Gussmann P, Schusdziarra V. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab. 2004. 89:3048–3054.

26. Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, Boyadjian R. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. 2002. 87:3997–4000.

27. Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr. 1998. 68:794–801.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download