This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The purpose of this study was to evaluate the treatment outcome of our optic nerve sheath meningioma (ONSM) case series in terms of preventing tumor growth and preserving vision in ONSM patients.
Methods
Between July 2003 and March 2015, 1,398 patients with intracranial meningioma were diagnosed at Seoul National University Bundang Hospital. Among them, only 13 patients (0.93%) were diagnosed with ONSM and enrolled in the present study. Tumor volume changes of ONSM patients and their visual acuity were evaluated before and after treatments.
Results
The median follow-up time was 50 months (range, 12–133 months). Visual acuity was evaluated in 12 of 13 patients, and visual acuity was found to be preserved in 9 of 12 patients (75%). Tumor volume was reduced in all patients. The tumor control rate was 100% in the present study. The difference in tumor volume between pretreatment and last follow-up was statistically significant (p=0.015).
Conclusion
Intensity-modulated radiotherapy (IMRT) and gamma knife radiosurgery (GKS) could maintain visual acuity and stabilize tumor volume in ONSM patients, suggesting that IMRT and GKS may be effective therapies for ONSM. However, which treatment is the more effective modality must be confirmed by prospective studies and longer-term follow-up.
Go to :

Keywords: Optic nerve, Meningioma, Visual acuity, Tumor volume, Stereotactic radiosurgery
INTRODUCTION
Optic nerve sheath meningioma (ONSM) originates from meningothelial cap cells in the arachnoid membrane that surrounds the optic nerve [
12345]. ONSM is a relatively rare tumor and represents 1–2% of all intracranial meningiomas [
46789]. A classification system for ONSM according to the tumor location has been established by Schick et al. [
310]. This classification divides ONSM into three main types: type I, purely intraorbital lesions; type II, intraorbital ONSM with extension through the optic canal or superior orbital fissure; and type III, intraorbital lesions with widespread intracranial tumor extension (>1 cm).
The majority of ONSM are benign tumors. Symptoms of ONSM include painless loss of vision, proptosis, visual field defect, afferent pupillary defect, color vision disturbance, optic disc edema, and ocular motility disturbance [
311]. Among these symptoms, painless loss of vision is the most serious problem. Although the incidence of tumor-associated mortality is relatively low, almost all patients experience gradual or rapid visual loss without any intervention, eventually losing vision in the affected eye and jeopardizing the normal contralateral vision.
Observation, surgery and/or radiotherapy are the main strategies in the management of ONSM [
161213]. A wait-and-see strategy had been recommended for asymptomatic patients with stable or slowly growing tumors [
113]. Several articles have focused on the use of surgery in ONSM patients. Because of the circumferential relationship with the optic nerve and its vascular supply, surgery always resulted in postoperative blindness [
8914]. Therefore, surgery might be considered in selective cases as a purpose of decompression in patients with optic chiasm compression.
Recently, radiation therapy has been recommended as a treatment of choice for patients with ONSM [
146151617]. According to a previous report, intensity-modulated radiotherapy (IMRT) leads to tumor growth control and visual stabilization [
181920].
In this retrospective study, we evaluated the treatment outcome of ONSM patients to determine which modality is appropriate to maintain vision and control tumor growth.
Go to :

MATERIALS AND METHODS
Between July 2003 and March 2015, 1,398 patients with intracranial meningioma were diagnosed at the authors' institution. Sixteen (1.14%) patients had radiographically confirmed ONSM, and 3 patients were lost to follow-up. Eventually, the remaining 13 (0.93%) patients were enrolled in the present study. All patients had a complete assessment by an ophthalmologist, including visual acuity (Snellen chart) and visual fields (Goldmann visual field or Humphrey visual field testing). After treatment, patients were followed-up with basic tests to evaluate their visual acuity and change of tumor volume. Presented study was approved by an Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1701-379-109).
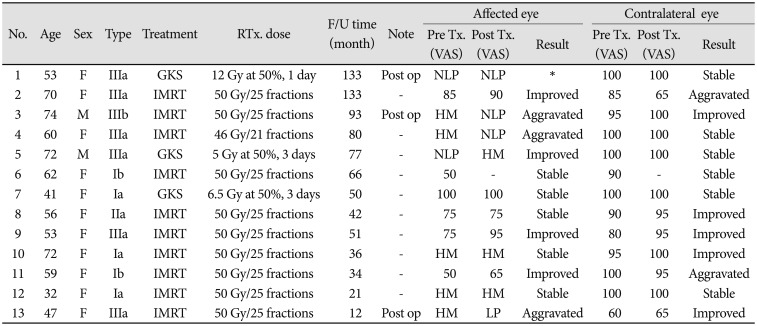
Thirteen patients with 13 unilateral ONSM were treated with IMRT or gamma knife radiosurgery (GKS). No one had died of ONSM. We used to apply with IMRT. Using a thermoplastic mask, baseplate fixation, and three-point laser-alignment to immobilized patients. We also used orbit magnetic resonance and stimulation computer tomography planning, the thickness was 1 mm and 2 mm. Gross tumor volume (GTV) covered all gross disease. GTV was delineated using contrast-enhanced simulation CT scan and gadolinium-enhanced MRI fusion. Clinical target volume (CTV) was usually identical to the GTV or covered additional microscopic spread beyond gross disease. Planning target volume was defined as the CTV with a margin of 5 mm. Ten patients were treated by IMRT with a dose of 50 Gy in 25 fractions, administered as primary treatment in 7 patients, after surgery in 2 patients and 1 patient received a dose of 46 Gy in 21 fractions as primary treatment (
Table 1).
Table 1
Characteristics of patients
|
No. |
Age |
Sex |
Type |
Treatment |
RTx. Dose |
F/U time (month) |
Note |
Affected eye |
Contralateral eye |
|
Pre Tx. (VAS) |
Post Tx. (VAS) |
Result |
Pre Tx. (VAS) |
Post Tx. (VAS) |
Result |
|
1 |
53 |
F |
IIIa |
GKS |
12 Gy at 50%, 1 day |
133 |
Post op |
NLP |
NLP |
* |
100 |
100 |
Stable |
|
2 |
70 |
F |
IIIa |
IMRT |
50 Gy/25 fractions |
133 |
- |
85 |
90 |
Improved |
85 |
65 |
Aggravated |
|
3 |
74 |
M |
IIIb |
IMRT |
50 Gy/25 fractions |
93 |
Post op |
HM |
NLP |
Aggravated |
95 |
100 |
Improved |
|
4 |
60 |
F |
IIIa |
IMRT |
46 Gy/21 fractions |
80 |
- |
HM |
NLP |
Aggravated |
100 |
100 |
Stable |
|
5 |
72 |
M |
IIIa |
GKS |
5 Gy at 50%, 3 days |
77 |
- |
NLP |
HM |
Improved |
100 |
100 |
Stable |
|
6 |
62 |
F |
Ib |
IMRT |
50 Gy/25 fractions |
66 |
- |
50 |
- |
Stable |
90 |
- |
Stable |
|
7 |
41 |
F |
Ia |
GKS |
6.5 Gy at 50%, 3 days |
50 |
- |
100 |
100 |
Stable |
100 |
100 |
Stable |
|
8 |
56 |
F |
IIa |
IMRT |
50 Gy/25 fractions |
42 |
- |
75 |
75 |
Stable |
90 |
95 |
Improved |
|
9 |
53 |
F |
IIIa |
IMRT |
50 Gy/25 fractions |
51 |
- |
75 |
95 |
Improved |
80 |
95 |
Improved |
|
10 |
72 |
F |
Ia |
IMRT |
50 Gy/25 fractions |
36 |
- |
HM |
HM |
Stable |
95 |
100 |
Improved |
|
11 |
59 |
F |
Ib |
IMRT |
50 Gy/25 fractions |
34 |
- |
50 |
65 |
Improved |
100 |
95 |
Aggravated |
|
12 |
32 |
F |
Ia |
IMRT |
50 Gy/25 fractions |
21 |
- |
HM |
HM |
Stable |
100 |
100 |
Stable |
|
13 |
47 |
F |
IIIa |
IMRT |
50 Gy/25 fractions |
12 |
Post op |
HM |
LP |
Aggravated |
60 |
65 |
Improved |

Three patients underwent GKS. Among these patients, two patients underwent GKS as primary treatment, one patient's dose was 5 Gy in 3 fractions at the 50% isodose line (total dose of 30 Gy), and the other patient's dose was 6.5 Gy in 3 fractions at the 50% isodose line (total dose of 39 Gy). Treatment fractions were administered every other day. Another patient underwent GKS after surgery with a single-fractionation dose of 12 Gy at the 50% isodose line (total dose of 24 Gy) (
Table 1).
The change of tumor volume and visual acuity in ONSM patients were evaluated before and after treatments. Three patients underwent operations before radiotherapy. The purpose of the operation was to decompress the optic chiasm to protect the vision of the contralateral side. The portion of the ONSM in the optic chiasm was removed, and the intraorbital portion of the ONSM was left for radiation treatment.
The best-corrected Snellen visual acuity was used at a distance of 5 meters in 200 lux of light. We applied American Medical Association guidelines to transform visual acuity to a visual acuity score (VAS) system. The VAS ranged from 0 (visual acuity=0.01, near blindness) to 100 (visual acuity=1.0, normal). However, we could not apply hand motion (HM), light perception (LP), and no light perception (NLP) with the VAS system. Schulze-Bonsel et al. [
21] supposed mean visual acuity of HM was 0.005 and no results were obtainable for LP. Therefore, these levels of visual perception were marked as the original form. Each patient's VAS was compared between preradiotherapy and last follow up results. We defined improvement or aggravation if the change of VAS was greater than or equal to 5 points.
The tumor volume was measured with 3D volumetry. Using the INIFINITT Picture Archiving and Communication System (PACS) program (INFINITT healthcare Co. Ltd, Seoul, Korea), the 2D ONSM area from MRI scans were multiplied by the sum of the slice thickness and slice interval. Individual patient tumor volume was measured on MRI by a single author. The radiographic outcomes were compared between pretreatment and last follow-up, as well as between preradiotherapy and last follow-up.
Statistical analysis
The comparison of tumor volume between pretreatment and last follow-up carried out using the paired t-test. These tests were performed by using SPSS statistical software (ver 23, IBM Corp., Armonk, NY, USA). p<0.05 was considered significant.
Go to :

RESULTS
Patients' characteristics
Patients' median age at the time of treatment was 59 years (range 32–74 years). The male-to-female ratio was 1:5.5 (2 males to 11 females). According to the radiographic findings, the tumor was located on the right side in 4 patients and on the left side in 9 patients. All patients unilaterally affected. Three of the ONSM patients showed intraorbital fusiform expansion (Type Ia), and 2 patients demonstrated intraorbital tubular expansion (Type Ib). One patient demonstrated intracanalicular extension (Type IIa). Six of the patients exhibited wide-spread intracranial tumor extension up to the chiasm (Type IIIa), and one to the contralateral side (Type IIIb). Median follow-up time was 50 months (range, 12–133 months). Ten patients were treated with IMRT and three patients were treated with GKS appropriately.
Outcome
The tumor volume was measured at pretreatment and last follow-up. The pretreatment tumor volumes ranged from 288.7 mm3 to 4,987.6 mm3, and the mean tumor volume was 2,083.8 mm3. Thirteen patients' tumor volume was reduced on the last follow-up MRI examination compared with the initial pretreatment MRI examination. The mean tumor volume for these patients decreased from 2,083.8 mm3 to 1,400.8 mm3 (range, 243.3–3,244.5 mm3). The difference in tumor volume between pretreatment and last follow-up was statistically significant (p=0.015, t-test). In addition, there was no evidence of tumor progression or recurrence in our patients. Hence, the tumor control rate was 100% in the present study.
Two kinds of treatments were used in this series. The tumor volume of the 10 patients who underwent IMRT was compared between preradiotherapy and last follow-up, which showed a significant difference (
p<0.003). Because of the small number of patients who underwent GKS, we did not compare the tumor volume of these patients. The change of tumor volumes from ONSM patients was summarized in
Table 2.
Table 2
Summary of the change of tumor volume
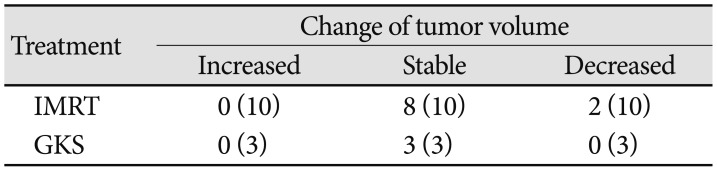
|
Treatment |
Change of tumor volume |
|
Increased |
Stable |
Decreased |
|
IMRT |
0 (10) |
8 (10) |
2 (10) |
|
GKS |
0 (3) |
3 (3) |
0 (3) |

Changes in visual acuity
Another point in the treatment of ONSM was a vision. Two patients were NLP before they got radiation treatment. Number 5 patient became NLP within 3 months. However, number 1 patient turned into NLP more than 3 years before he was treated with GKS, and there was no potentiality of recovery. Therefore, number 1 patient was excluded from the comparison of VAS. Six patients' visual acuity of the affected side were very poor (less than 0.01) before treatment. Five patients' visual acuity were HM, and 1 patient's visual acuity was NLP. Among these 5 patients with HM, 2 patients continued perceiving HM, 2 patients developed NLP, and 1 patient developed LP. The other 1 patient improved to HM from NLP. In addition, the remaining 6 patients' VAS were better than 50 points. Half of the 6 patients' visual acuity were stable, and the others improved in VAS scores of 5, 15, and 20 points respectively. In general, visual acuity was preserved in 9 of 12 patients (75%). Visual acuity of the contralateral eye remained stable or improved in 11 patients (84.6%) and deteriorated in two patients of 5 and 20 points (
Table 3).
Table 3
The changes of visual acuity in optic nerve sheath meningioma patients
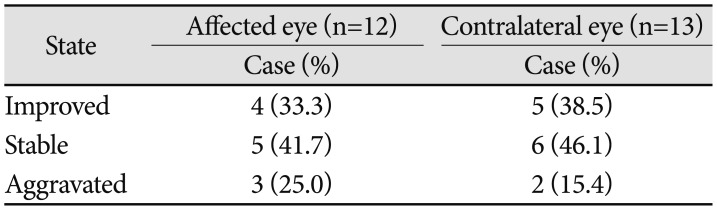
|
State |
Affected eye (n=12) |
Contralateral eye (n=13) |
|
Case (%) |
Case (%) |
|
Improved |
4 (33.3) |
5 (38.5) |
|
Stable |
5 (41.7) |
6 (46.1) |
|
Aggravated |
3 (25.0) |
2 (15.4) |

Two types of radiation were used to manage ONSM patients: IMRT and GKS. In 10 patients who underwent IMRT, visual acuity stabilized and improved in 7 patients (70%), deteriorated in 3 patients (10%). Three patients underwent GKS. Two patients' visual acuity was stabilized or improved, and 1 patient was excluded from comparisons (
Table 4).
Table 4
The changes of affected eye vision in different treatment modality
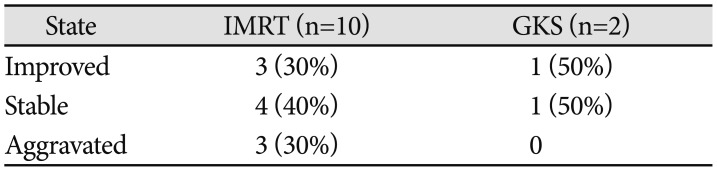
|
State |
IMRT (n=10) |
GKS (n=2) |
|
Improved |
3 (30%) |
1 (50%) |
|
Stable |
4 (40%) |
1 (50%) |
|
Aggravated |
3 (30%) |
0 |

Radiation-induced toxicity
Acute toxicity was mild in a small number of patients. Nausea was observed in 3 patients, dry eye was found in one patient and mild headache appeared in 2 patients. Chronic toxicity occurred in 1 patient with diabetes mellitus, in whom radiation-induced retinopathy was observed 9 months after IMRT performed.
Go to :

DISCUSSION
ONSM is a relatively rare tumor. Although the incidence of tumor-associated mortality is relatively low, almost all patients experience visual deterioration eventually. Management of ONSM might be a challenge to clinicians. The best treatment for patients with ONSM remains controversial.
According to reports on the use of surgery for ONSM, surgery has been associated with inevitable dissection of the vascular supply of the optic nerve [
14816]. This damage of the vascular supply to the optic nerve during the procedure led to ONSM patients consequently presenting blindness [
4]. In Dutton's review [
22], among 120 patients with primary ONSM treated with surgery alone, only 5% gained visual improvement, while 94% experienced loss of vision. Although surgery may lead to blindness, it is necessary in cases of intracranial extension to ameliorate severe disfiguring proptosis or to prevent contralateral optic nerve involvement [
8]. In the present study, 3 (23%) patients with ONSM underwent surgery before adjuvant treatment to prevent contralateral optic nerve and chiasm involvement.
There are two main goals in the treatment of ONSM: first to maintain or improve vision, and second to control tumor growth. Recently, radiotherapy has been recommended as a treatment of choice for patients with ONSM [
61517]. Solda et al. [
14] summarized dozens of articles and found that favorable visual outcome (improved or stable) was reported in 83–100% of patients, the local control rate of ONSM was 100%, and the range of total doses used was 45–50.4 Gy. In another review article, author summarized that several IMRT papers reporting improved and stabilized visual outcome in 100% of patients and tumor control in 100% [
4]. In the present study, the rate of improved or stable visual outcomes in our 13 patients was in 87%, and that of tumor control was 100%. Among 10 patients who underwent IMRT, visual acuity stabilized in 7 patients (70%) and deteriorated in 3 patient (30%). The tumor control rate was 100% in these 10 patients. The comparison of tumor volume showed a significant difference between preradiotherapy and last follow-up (
p<0.003). The results of our study are consistent with other reports and indicate that IMRT appeared to be an effective treatment for the management of ONSM.
GKS is another treatment modality used in the management of ONSM patients. We also attempted to treat ONSM patients with GKS. Within the study period only 3 patients underwent GKS. The advantages of GKS are that the delivery method achieves a much higher degree of target conformity than conventional radiotherapy techniques while minimizing radiation exposure to surrounding normal tissues, but a disadvantage of GKS is that effective treatment doses cannot be tolerated by the optic nerve [
4]. In the treatment of ONSM patients, clinicians must weigh the pros and cons of GKS. Recently published case series included small numbers of patients. One study with 30 patients reported that 24 (80%) patients were improved or stable after treatment with GKS. The rate of tumor control in this report was 93%, regression was 67%, and progression was 7%.
Three patients were treated with GKS in the present study. Except for one patient with blindness after surgery, another patient's visual acuity was improved, the other patient's visual acuity was stabilized, and no patient experienced tumor progression. Based on these results, GKS appears to be an effective tool for treating patients with ONSM. However, the number of patients was small, and statistical significance could not be assessed between the therapeutic outcomes of GKS and IMRT.
Based on our experience and the data of this study, we expected that when visual acuity was lower than 0.01, there was no hope of improving visual acuity to >0.01. Therefore, we could focus more attention on attempting to protect vision on the contralateral side in these patients. However, when we diagnosed patients with ONSM and their visual acuity was better than 0.01, according to our data, visual acuity remained stable in half of 6 patients and was improved in the rest of 6 patients improved 5, 15, and 20 points respectively. We could apply some aggressive treatment in such patients. The wait-and-see strategy might need to reconsider in this kind of patients.
The importance of contralateral-side vision cannot be neglected. In this study, 11 of 13 patients maintained their visual acuity by appropriate treatment. However, 2 patients suffered from deterioration of visual acuity, with VAS falling from 85 to 65, and 100 to 95 points. The reason for the deterioration in number 2 patient was not caused by radiotherapy. However, the other one was not clear. In general, the outcome of contralateral side vision was acceptable in the treatment of ONSM patients.
As mentioned above, several articles have shown tumor control rates of 100%, as in the present study. Tumor volume significantly differed between pretreatment and last follow-up, with p=0.015. Apparently, tumor control needs no longer the focus of treatment in ONSM patients, and we supposed to redirect attention to improving or preserving impaired vision.
In conclusion, IMRT and GKS could maintain visual acuity of the affected and contralateral eyes and stabilized tumor volume in ONSM patients, suggesting that IMRT and GKS may be effective therapies for ONSM. Some patients presented chronic radiation therapy toxicities such as radiation retinopathy, and we should pay caution to avoid these side effects. The optimal modality should be confirmed by prospective studies and longer-term follow-up.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download