Abstract
Surgery, anticoagulation therapy, pregnancy, and hormone treatments, such as bromocriptine, are well-characterized precipitating factors for pituitary apoplexy. However, whether cytotoxic chemotherapy for systemic cancer could cause pituitary apoplexy has not been investigated. Here, we present a case of a 41-year-old woman who developed a severe headache with decreased visual acuity after intravenous cytotoxic chemotherapy to treat metastatic breast cancer. Preoperative neuroimaging revealed pituitary adenoma with necrosis. Operative findings and pathologic examination concluded extensive necrosis with a small intratumoral hemorrhage in a pre-existing pituitary adenoma. We reviewed two additional previously published cases of pituitary apoplexy after systemic chemotherapy and suggest that cytotoxic chemotherapy may induce pituitary apoplexy.
Pituitary apoplexy is hemorrhage or infarction of a pituitary gland tumor presented as acute onset of headache, ophthalmoplegia, and visual impairment. Pituitary apoplexy is an endocrine and surgical emergency that requires prompt treatment with glucocorticoid replacement and surgical decompression, especially when accompanied by visual symptoms or mental status changes. Known precipitating factors of pituitary apoplexy include surgery, anticoagulation therapy, and post-partum hemorrhage [1]. Additionally, bromocriptine, cabergoline, and other pituitary-stimulating treatments, such as gonadotropin-releasing hormone (GnRH) agonist or luteinizing hormone-releasing hormone (LHRH) agonist, have been reported to cause apoplexy [23]. Most cases of pituitary apoplexy occur in the absence of any precipitating factor. Cytotoxic chemotherapy for systemic cancer has not been considered as a cause of apoplexy; however, there have been two previously reported cases of pituitary apoplexy following systemic chemotherapy [45].
A 41-year-old woman diagnosed with metastatic breast cancer presented to an emergency room with headache and vomiting a few hours after her third cycle of intravenous cytotoxic chemotherapy with doxorubicin and cyclophosphamide. Her breast cancer was confirmed following modified radical mastectomy of her right breast 10 weeks prior. Pathologic examination revealed stage Ib infiltrative ductal carcinoma. She was prescribed intravenous treatment with doxorubicin and cyclophosphamide every three weeks. Her past medical history included menstrual irregularity, but she did not consider it unusual and no work up was performed. After neurological examination in the emergency room, she was prescribed analgesics and anti-emetics and sent home.
The following day, she woke up with diplopia and visual disturbances. She returned to the emergency room after having a brain computed tomography (CT) scan at a nearby hospital to rule out intracranial hemorrhage. The CT scan revealed an enlarged sella containing a slightly low-density, round mass 2.8 cm in diameter (Fig. 1A). Upon arrival at the emergency room, she was determined to have left sixth-cranial nerve palsy following a hand motion in the left-eye visual acuity test and temporal hemianopsia in the right-eye visual field test (Fig. 2A). Her initial sodium concentration was 138 mmol/L and there was no definite sign of adrenal insufficiency. Basal hormone test results were as follows: growth hormone, 19.32 ng/mL (normal: 0.38–12.00); cortisol, 66.5 µg/dL (normal: 5.3–22.5); T3, 0.78 ng/mL (normal: 0.60–1.81); free T4, 1.23 ng/dL (normal: 0.89–1.76); thyroid stimulating hormone, 0.70 µIU/mL (normal: 0.55–4.78); luteinizing hormone, 0.8 mIU/mL (normal: 1.0–95.6); follicle stimulating hormone, 11.4 mIU/mL (normal: 1.7–21.5); and estrogen, 30.3 pg/mL (12.4–341). Magnetic resonance imaging (MRI) confirmed a 2.8-cm mass with solid and cystic portions with spotty intratumoral hemorrhage (Fig. 1B) extending from an enlarged pituitary fossa to suprasellar cistern and left cavernous sinus (Fig. 1C).
A transsphenoidal approach (TSA) and tumor removal was performed the following day. After dura incision, necrotic material flowed out and a mass with a blood clot was found upon microsurgical viewing (Fig. 3). Pathology of the specimen revealed pituitary adenoma with extensive mixed-stage (recent and remote) necrosis (Fig. 4). At the end of decompression, a frozen biopsy confirmed a flesh-firm normal gland at the bottom of the surgical field. A post-operative MRI, taken 30 hours after surgery, demonstrated that gross total resection was achieved while saving the normal gland as a posteriorly linear enhancing portion in the sagittal view.
The patient felt improved visual acuity immediately postoperative and developed transient diabetes insipidus on the first postoperative day that was treated with desmopressin and fluid replacement. Three days postoperative, her left sixth-cranial nerve palsy recovered. One week later, she was discharged following tamoxifen chemotherapy. Three days after discharge, she was again admitted to the emergency department due to cerebrospinal fluid rhinorrhea and severe headache. A brain CT scan performed at an outside hospital showed pneumocephalus throughout the brain. After three days of lumbar drainage, the patient continued to suffer from posterior nasal drip. Thus, TSA revision was performed and the sella floor was enforced with bi-layered lateral oblique muscle fascia. Three months after the initial surgery, the patient was doing well. Ophthalmologic examination at the 3-month follow-up revealed improved visual acuity of the left eye up to 0.2 and normalization of the right-eye visual field defect (Fig. 2B).
There are several predisposing conditions for pituitary adenoma, such as coronary artery surgery, pregnancy, head trauma, and gamma knife irradiation [16]. Hormonally active tumors, such as acromegaly and Cushing's disease, or large non-functioning tumors are vulnerable to apoplexy. Bromocriptine/cabergoline and GnRH agonist/LHRH agonist are well-characterized precipitating treatments that are used to treat prolactinoma and prostate cancer, respectively [23]. However, most pituitary apoplexy cases lack any known predisposing condition.
Semple et al. [1] analyzed consecutive pituitary apoplexy cases from a single institute; among 38 pituitary apoplexy cases, only nine cases (24%) had a precipitating factor(s). In the group with a precipitating factor, a larger portion had an impaired level of consciousness at the time of presentation than in the group without any precipitating factor. Most patients with a precipitating factor were also more likely to have a hemorrhage or hemorrhagic infarction than patients without a precipitating factor.
Biousse et al. [6] similarly analyzed 30 consecutive cases of pituitary apoplexy from a single institution. Potential risk factors, including anticoagulation, surgery, post-partum, and bromocriptine therapy, were identified in nine cases (30%). Furthermore, these patients with associated conditions had a higher frequency of altered mental status at the time of presentation and more neuro-ophthalmic sequelae after treatment than the patients without risk factors [6].
To our knowledge, only two cases of pituitary apoplexy related to cytotoxic chemotherapy have been reported [45]. Pituitary apoplexy was reported in the second cycle of cisplatin, methotrexate, and vinblastine chemotherapy in a 70-year-old man with penile squamous cell carcinoma [5]. The patient had sudden, severe headache and diplopia due to left third-nerve palsy. He made good progress upon conservative management with steroids alone and the third-nerve palsy gradually resolved. The other patient, a 55-year-old man, presented with a severe headache, photophobia, and nausea on day 6 following induction chemotherapy for acute myeloid leukemia (AML) [4]. The patient had thrombocytopenia and panhypopituitarism. The headache resolved after replacement therapy with hydrocortisone and testosterone.
In our case, pituitary apoplexy occurred after the third cycle of doxorubicin and cyclophosphamide in a 41-year-old woman with metastatic breast cancer. She presented with severe headache, nausea, diplopia, left sixth-cranial nerve palsy, and decreased visual acuity, which necessitated emergency decompression via TSA. Thus, our case is the only pathologically proven pituitary apoplexy among those related to systemic chemotherapy.
The timing of pituitary apoplexy and administration of systemic chemotherapy was not consistent between the three patients. In the AML case, the symptoms arose during the first cycle of chemotherapy (day 6 of induction chemotherapy). In the penile cancer case, the patient suffered a headache after the second cycle. In our case, the patient had sudden visual symptoms 1 day after the third cycle of chemotherapy, and we could find multi-stage necrosis in the surgical specimen of the pituitary adenoma. Based on hormone study, all three cases of pituitary apoplexy following cytotoxic chemotherapy were non-functioning pituitary adenomas.
We suggest that when an incidental large pituitary adenoma is identified in patients with systemic cancer who are to be treated with cytotoxic chemotherapy, prophylactic pituitary adenoma removal should be considered to prevent pituitary apoplexy.
Figures and Tables
Fig. 1
Preoperative neuro-images. (A) Pre-operative brain computed tomography scan demonstrating a lobulated, contoured, low-density lesion on the widened sella. There was no evidence of either intracranial or intratumoral hemorrhage. Pre-operative magnetic resonance imaging of T2 axial (B) and T1 weighted (C), gadolinium-enhancement, coronal views showing lobulated, contoured, large, mixed solid and necrotic masses with spotty intratumoral hemorrhage (white arrow) and suprasellar extension.
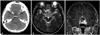
Fig. 2
Pre- and post-operative visual field examinations. A: Visual field tests were not possible on the left eye. Lateral and inferior medial three-quadrant anopsia was found preoperatively. B: Postoperative 2-month visual field test revealed nearly complete recovery of the right eye and left-eye temporal hemianopsia.

Fig. 3
Intra-operative picture showing necrotic material flowing out after dura incision (A) and a mass with blood clot (white arrow) (B).

Fig. 4
Pituitary adenoma showing varying degree of necrosis (hematoxylin and eosin staining, ×400). Some areas retain papillary tumor configuration which is readily discernable as pituitary adenoma, although the tumor shows pyknotic nuclei and acidophilic cytoplasm (A) and complete necrosis of tumor cells in this area, showing ghosty cells (B).

References
1. Semple PL, Jane JA Jr, Laws ER Jr. Clinical relevance of precipitating factors in pituitary apoplexy. Neurosurgery. 2007; 61:956–961.

2. Guerrero-Pérez F, Marengo AP, Planas-Vilaseca A, Flores-Escobar V, Villabona-Artero C. Pituitary apoplexy induced by triptorelin in patient with prostate cancer. Endocrinol Nutr. 2015; 62:411–412.

3. Chng E, Dalan R. Pituitary apoplexy associated with cabergoline therapy. J Clin Neurosci. 2013; 20:1637–1643.

4. Silberstein L, Johnston C, Bhagat A, Tibi L, Harrison J. Pituitary apoplexy during induction chemotherapy for acute myeloid leukaemia. Br J Haematol. 2008; 143:151.

5. Davies JS, Rees DA, Evans LM, Scanlon MF. Pituitary apoplexy following combination chemotherapy-a case report. Endocr Relat Cancer. 1998; 5:151–153.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download