Abstract
Intermediate pilomyxoid tumors (IPTs) were defined by the presence of some features typical of pilomyxoid astrocytoma (PMA) in combination with features that could be considered more consistent with pilocytic astrocytoma (PA). PMA is rare in the cerebellum. And, IPT in the cerebellum is rarer than PMA. To our knowledge, only 2 reports have described IPT in the cerebellum. A 5-year-old boy had nausea and vomiting. Computed tomography revealed a large, round, low-density tumor in the cerebellar vermis area. On enhanced magnetic resonance imaging (MRI), the tumor showed inhomogeneous diffuse enhancement; the central portion showed homogenous enhancement, while the peripheral portion showed inhomogeneous enhancement. The patient underwent a midline suboccipital craniotomy, and gross total resection was performed. The tumor was gray-colored, rubbery hard, and severely hemorrhagic with a clear boundary. On pathologic examination, the combined features of both PA and PMA were retrospectively indicative of an IPT. The patient was symptom-free for 18 months, with no evidence of tumor recurrence on MRI. More observation and further studies on PMA and IPT are required to determine the most appropriate treatment for these tumors.
Intermediate pilomyxoid tumors (IPTs) were defined by the presence of some features typical of pilomyxoid astrocytoma (PMA) in combination with features that could be considered more consistent with pilocytic astrocytoma (PA) [1]. IPT belongs to the spectrum of PMA. PMA is usually located in the hypothalamic-chiasmatic area [12]. IPT is similar to PMA [1]. PMA in the hypothalamic-chiasmatic area exhibits increased biological aggressiveness relative to PA [34]. PMA is rare in the cerebellum [15678]. However, IPT in the cerebellum is rarer than PMA. To our knowledge, only 2 reports have described [69]. Thus, the clinical aspect of IPT is not entirely known. We report a case of IPT in the cerebellum of a 5-year-old boy at our institution.
A 5-year-old boy presented in our department with a 5-day history of nausea and vomiting. He had no past history of disease, even during the peri- or neonatal period. Neurologic examination showed no deficits. Cerebellar function test was normal. Enhanced brain computed tomography (CT) revealed a 4.5-cm, large, round, bulging, low-density solid brain tumor with mild peritumoral edema at the cerebellar vermis area. Mild hydrocephalus was shown due to fourth ventricle compression by the tumor. The tumor showed multifocal inhomogeneous enhancement (Fig. 1). Magnetic resonance imaging (MRI) with gadolinium enhancement was performed. The tumor showed low signal intensity on T1 and high signal intensity on T2 imaging, with homogeneous signal intensity. On enhanced MRI, the tumor showed inhomogeneous diffuse enhancement; the central portion showed homogenous enhancement, while the peripheral portion showed inhomogeneous enhancement (Fig. 2). The tumor showed a high apparent diffusion coefficient value on diffusion MRI, and diffuse increased cerebral blood volume on perfusion MRI. Severe mass effect and hydrocephalus by the tumor were also observed. Brainstem invasion was not observed. Whole spine MRI revealed no evidence of metastasis or leptomeningeal dissemination (LD).
The patient underwent a midline suboccipital craniotomy. Following a combination of telovelar dissection and incision of the inferior one-third of the vermis, a gray tumor was encountered. The tumor was rubbery hard and severely hemorrhagic with a clear boundary. Internal debulking and piecemeal removal of the tumor were performed. Gross total resection (GTR) was performed. We checked the rhomboid fossa superiorly and the foramen Magendie caudally. There were no residual tumors, and the patient recovered completely with no neurological deficits.
Histological examination of the resected specimen revealed a moderately cellular tumor composed of monomorphous cells (Fig. 3A). The tumor cells occasionally showed long cytoplasmic processes, but some oligodendroglioma-like tumor cells with perinuclear halos and distinct cytoplasmic borders were also observed (Fig. 3B). Some areas displayed angiocentric arrangement of the tumor cells (Fig. 3C). Microcystic changes were present, but myxoid stroma was absent. Moreover, very few Rosenthal fibers and eosinophilic granular bodies were identified. Histological features of a high-grade glioma, such as microvascular proliferation or pseudopalisading necrosis, were absent. Immunohistochemistry of glial fibrillary acidic protein showed strong reactivity in the tumor cells (Fig. 3D). The Ki-67 labeling index was less than 1%. Immunohistochemical staining of neuronal markers, including synaptophysin, chromogranin, or neurofilament protein, was negative. Overall, the microscopic findings indicated a category of tumors somewhere between PA and PMA. The pathological diagnosis of IPT was rendered.
In 2010, Johnson and his colleagues [1] reported a spectrum of PMA: IPT. There were 42 IPTs. Intermediate lesions were defined by the presence of some features typical of PMA in combination with features that would be considered more consistent with PA. All intermediate lesions had some combination of pilomyxoid qualities, such as small monomorphous bipolar cells, focal myxoid substance, and variable angiocentric growth; and at least some pilocytic features, such as microcystic change, cells with thicker, longer processes, compact finely fibrillar tumor tissue, and Rosenthal fibers. Our case showed consistent findings considering these observations.
PMA is usually located in the hypothalamic-chiasmatic area, and has been found to have a more aggressive behavior than PA [23]. Komotar and his colleagues [3] reported that PMA had a higher rate of local recurrence (76%) and LD (14%) than PA, despite equal degrees of GTR. PMA also demonstrated shorter progression-free survival (26 months) and overall survival (63 months) than PA; 33% of patients with PMA died of their disease [3].
Information about PMA of the cerebellum is very little due to the very few reported cases. Only 10 cases of PMA in the cerebellum have been reported [59]. Although the prognosis of cerebellar PMA remains unknown, several authors have reported a more favorable outcome for cerebellar PMA than for PMA of other locations. This is because GTR or near total resection is more feasible for cerebellar PMA [5710]. El Beltagy and his colleagues [9] reported a recurrence rate of 66.7%, GTR rate of 8.3%, and LD of 75% in PMA of the cerebellum. The 5-year overall survival rate was 87.5%; however, this was observed in a combination of PMA and IPT. The mean interval to recurrence was 9 months. In IPT of the cerebellum, they reported a recurrence rate of 20%, GTR rate of 20%, and LD of 40% [9]. The results of the cerebellar IPT were more favorable than the results of the cerebellar PMA.
Until now, surgery is the best treatment; however, the GTR rate has been found to be affected by brainstem invasion. Almost all cases (95.5%) reported by El Beltagy and his colleagues [9] showed brainstem invasion. In other cases of GTR of cerebellar PMA, no recurrence has been reported [5710]. In contrast, among the 11 reported cases, only 2 cases (18.2%) have achieved GTR of cerebellar IPT [69]. Our case achieved GTR, without brainstem invasion.
PA is a grade I neoplasm according to the World Health Organization (WHO) classification of tumors [11]. In 2007, the WHO classification of tumors considered PMA as a WHO grade II neoplasm [12]. However, in 2016, the WHO classification of tumors has changed the grading of PMA. Suppression of the PMA grading has been suggested until further studies clarify their behavior [11]. More studies on PMA and IPT are required to more accurately determine the treatment and prognosis of these tumors.
We reported a case of intermediate pilomyxoid astrocytoma in the cerebellum of a 5-year-old boy. Surgery was performed to control the tumor. The patient was asymptomatic, and no evidence of recurrence was observed during the follow-up period. More observation and further studies on PMA and IPT are required to determine the most appropriate treatment for these tumors.
Figures and Tables
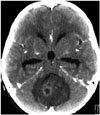 | Fig. 1A 5-year-old boy presented with a nausea and vomiting. Computed tomography showed a 4.5-cm large, bulging, round, low density, solid brain tumor with mild peritumoral edema at the cerebellar vermis area. Mild hydrocephalus was observed due to fourth ventricle compression by the tumor. The tumor showed multifocal inhomogeneous enhancement. |
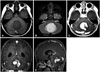 | Fig. 2Magnetic resonance imaging with gadolinium enhancement. A: The tumor had a low signal intensity on T1 imaging. B: The tumor had a high signal intensity with homogeneous signal intensity on T2 imaging. C: The tumor showed inhomogeneous diffuse enhancement on axial T1 imaging. D: The tumor showed inhomogeneous diffuse enhancement on coronal T1 imaging. E: The tumor showed inhomogeneous diffuse enhancement on sagittal T1 imaging. The central portion showed homogenous enhancement, while the peripheral portion showed inhomogeneous enhancement. |
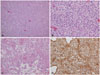 | Fig. 3A: The resected lesion comprised monomorphous tumor cells with moderate cellularity (hematoxylin and eosin staining, original magnification ×100). B: Some areas showed oligodendroglioma-like tumor cells with perinuclear halos and distinct cytoplasmic borders (hematoxylin and eosin staining, original magnification ×400). C: There was angiocentric arrangement of tumor cells around focally hyalinized vessels (hematoxylin and eosin staining, original magnification ×200). D: Immunohistochemistry of glial fibrillary acidic protein revealed strong positivity in the tumor cells with cytoplasmic processes (immunohistochemistry, original magnification ×200). |
References
1. Johnson MW, Eberhart CG, Perry A, et al. Spectrum of pilomyxoid astrocytomas: intermediate pilomyxoid tumors. Am J Surg Pathol. 2010; 34:1783–1791.
2. Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999; 58:1061–1068.

3. Komotar RJ, Burger PC, Carson BS, et al. Pilocytic and pilomyxoid hypothalamic/chiasmatic astrocytomas. Neurosurgery. 2004; 54:72–79. discussion 79-80.

4. Tsugu H, Oshiro S, Yanai F, et al. Management of pilomyxoid astrocytomas: our experience. Anticancer Res. 2009; 29:919–926.
5. Okano A, Oya S, Fujisawa N, et al. Significance of radical resection for pilomyxoid astrocytoma of the cerebellum: a case report and review of the literature. Childs Nerv Syst. 2013; 29:1375–1379.

6. Forbes JA, Mobley BC, O'Lynnger TM, et al. Pediatric cerebellar pilomyxoid-spectrum astrocytomas. J Neurosurg Pediatr. 2011; 8:90–96.

7. Ajani OA, Al Sulaiti G, Al Bozom I. Pilomyxoid astrocytoma of the cerebellum. J Neurosurg Pediatr. 2011; 7:539–542.

8. Nagaishi M, Yokoo H, Hirato J, Yoshimoto Y, Nakazato Y. Clinico-pathological feature of pilomyxoid astrocytomas: three case reports. Neuropathology. 2011; 31:152–157.

9. El Beltagy MA, Atteya MM, El-Haddad A, et al. Surgical and clinical aspects of cerebellar pilomyxoid-spectrum astrocytomas in children. Childs Nerv Syst. 2014; 30:1045–1053.

10. Fernandez C, Figarella-Branger D, Girard N, et al. Pilocytic astrocytomas in children: prognostic factors--a retrospective study of 80 cases. Neurosurgery. 2003; 53:544–553. discussion 554-5.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download