1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014; 16(Suppl 4):iv1–iv63. PMID:
25304271.

2. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D. Glioblastoma, IDH-wild-types. In : Louise DN, Brate DJ, Ohgaki H, editors. WHO Classification of Tumours of the Central Nervous System. Rev 4th ed. Lyon: IARC Press;2016. p. 28–45.
3. Dho YS, Jung KW, Ha J, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017; 5:16–23. PMID:
28516074.

4. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352:997–1003. PMID:
15758010.
5. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466. PMID:
19269895.
6. Marina O, Suh JH, Reddy CA, et al. Treatment outcomes for patients with glioblastoma multiforme and a low Karnofsky Performance Scale score on presentation to a tertiary care institution. J Neurosurg. 2011; 115:220–229. PMID:
21548745.

7. Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004; 6:227–235. PMID:
15279715.
8. Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011; 81:623–630. PMID:
20888136.

9. Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008; 9:29–38. PMID:
18082451.

10. McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009; 110:156–162. PMID:
18847342.

11. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655. PMID:
7165009.

12. Young J, Badgery-Parker T, Dobbins T, et al. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage. 2015; 49:258–264. PMID:
24996034.

13. Nayak L, DeAngelis LM, Brandes AA, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017; 19:625–635. PMID:
28453751.

14. Karayan-Tapon L, Quillien V, Guilhot J, et al. Prognostic value of O6-methylguanine-DNA methyltransferase status in glioblastoma patients, assessed by five different methods. J Neurooncol. 2010; 97:311–322. PMID:
19841865.

15. Palmisano WA, Divine KK, Saccomanno G, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000; 60:5954–5958. PMID:
11085511.
16. Kim J, Lee SH, Jang JH, Kim MS, Lee EH, Kim YZ. Increased expression of the histone H3 lysine 4 methyltransferase MLL4 and the histone H3 lysine 27 demethylase UTX prolonging the overall survival of patients with glioblastoma and a methylated MGMT promoter. J Neurosurg. 2017; 126:1461–1471. PMID:
27367247.

17. Provenzale JM, Ison C, Delong D. Bidimensional measurements in brain tumors: assessment of interobserver variability. AJR Am J Roentgenol. 2009; 193:W515–W522. PMID:
19933626.

18. Eng J. Receiver operating characteristic analysis: a primer. Acad Radiol. 2005; 12:909–916. PMID:
16039544.
19. Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010; 9:906–920. PMID:
20705518.

20. Jaspan T, Morgan PS, Warmuth-Metz M, et al. Response assessment in pediatric neuro-oncology: implementation and expansion of the RANO criteria in a randomized phase II trial of pediatric patients with newly diagnosed high-grade gliomas. AJNR Am J Neuroradiol. 2016; 37:1581–1587. PMID:
27127006.

21. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neurooncology working group. J Clin Oncol. 2010; 28:1963–1972. PMID:
20231676.

22. Chang SM, Wen PY, Vogelbaum MA, Macdonald DR, van den Bent MJ. Response Assessment in Neuro-Oncology (RANO): more than imaging criteria for malignant glioma. Neuro-Oncol Prac. 2015; 2:205–209.

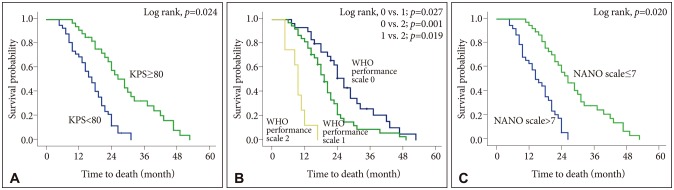
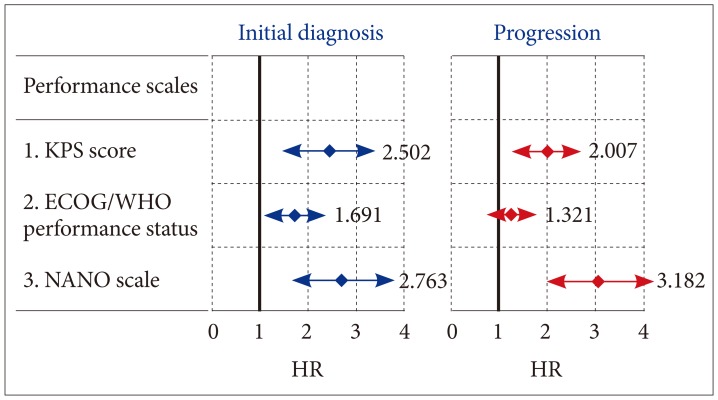

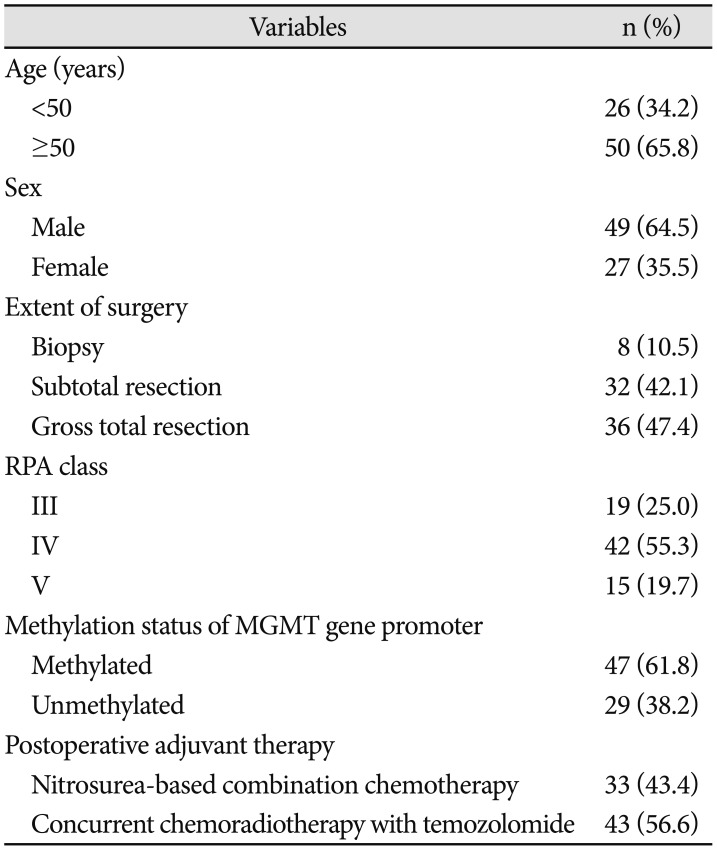
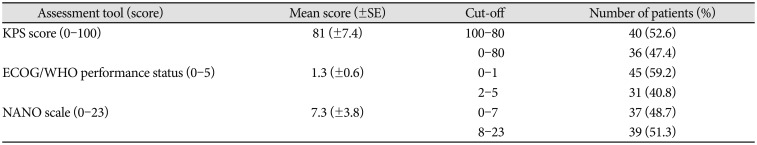




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download