Abstract
The incidence of leptomeningeal dissemination (LMD) of anaplastic glioma has been increasing. LMD can be observed at the time of initial presentation or the time of recurrence. As a result of both rarity and unusual presentation, a standard therapy has not yet been suggested. In contrast to leptomeningeal carcinomatosis for systemic solid cancers, a relatively prolonged survival is observed in some patients with LMD of anaplastic gliomas. Treatment modalities include whole craniospinal irradiation, intra-cerebrospinal fluid (CSF) chemotherapy, and systemic chemotherapy. In some cases, response to temozolomide (TMZ), with or without combined radiation has been reported. Here, we report two cases of LMD of an anaplastic glioma. In one case LMD presented at the time of diagnosis, and in the other at the time of recurrence after radiation. CSF cytology was positive in both cases, and persisted in spite of intrathecal methotrexate chemotherapy. Later, TMZ was prescribed for progressing brain parenchymal lesions, and both radiological and cytological responses were obtained after oral TMZ treatment.
Presentation of leptomeningeal dissemination (LMD) is either primary, without parenchymal lesions, or with recurrence, with or without parenchymal lesions. The distribution of LMD in gliomas is also variable and can include the brain leptomeningeal space only, the spinal cord surface only, or both [1]. The incidence of leptomeningeal metastases in anaplastic gliomas (≥WHO grade III) has been reported variably [2]. In accord with the rarity of LMD and with differences in clinical settings, treatments are chosen by the physician based upon previous treatment history, treatment availability, and patient compliance. Although statistical validation has not been achieved, treatment of primary LMD of anaplastic gliomas with temozolomide (TMZ) has yielded a remarkable response and occasional long-term survival [13]. This response was supported by a human cerebrospinal fluid (CSF) pharmacokinetic study, which showed relatively higher CSF penetration by TMZ [4].
In one of the cases presented herein, LMD was observed at the initial presentation in the conus medullaris with parenchymal tumor in a male patient without corresponding symptoms. In the other case, a female patient with a history of thalamic glioma treated by radiation only without biopsy, presented with LMD as multiple lesions along the cerebral aqueduct without specific symptoms. Here, we summarized our rare cases of LMD of anaplastic gliomas that showed cytological and radiological response to TMZ.
In 2012, a 54-year-old man visited a general hospital in Australia due to seizures, and he had received a craniotomy for resection of a left frontal lesion and a stereotactic biopsy of a cerebellar lesion. With a diagnosis of anaplastic astrocytoma with LMD, he visited our hospital. Preoperative brain CT images revealed a low-density mass with central foci of calcification at the left frontal premotor area (Fig. 1A). Postoperative magnetic resonance image (MRI) showed multiple infiltrative cerebellar lesions (no tumor on the biopsy at Australia) and hydrocephalus (Fig. 1B). Spine MRI demonstrated diffuse, nodular leptomeningeal enhancement around the thoracolumbar spinal cord, conus medullaris, and filum terminale (Fig. 1C), and the CSF cytology was positive for malignant cells. Postoperative MRI showed a residual enhancing lesion on the left frontal lobe (Fig. 1D). The second craniotomy was performed on March 15, 2012, and the pathologic diagnosis was WHO Grade III anaplastic oliogoastrocytoma of the left frontal lesion (Fig. 2). There was no 1p/19q chromosomal co-deletion. Later, our pathologist re-judged the tumor as a matglioblastoma isocitrate dehydrogenase (IDH)-wild type, according to the 2016 WHO new classification of gliomas [5].
The radiation oncologist judged that the patient already had LMD and refused to give local brain radiation. He instead administered adjuvant ifosphamide, carboplatin, and etoposide (ICE) chemotherapy [6]. After three cycles of the ICE regimen, the patient presented with gait disturbance and cognitive dysfunction. Increased intracranial pressure (ICP) on CSF examination (>25 cm CSF), markedly elevated CSF protein levels, and a delayed flow of CSF on radioisotope cisternography indicated hydrocephalus due to LMD. After an Ommaya reservoir insertion, ventriculolumbar perfusion (VLP) methotrexate chemotherapy was started [7]. After three VLP cycles, symptoms related to hydrocephalus had not changed, despite a decrease in ICP and negative CSF cytology conversion. The patient required a ventriculoperitoneal shunt, and after shunt insertion he showed mild improvement of cognitive dysfunction and gait disturbance. However, despite intraventricular chemotherapy, follow-up MRI showed marked progression of LMD with scattered parenchymal enhancing masses alongside the ventricle (Fig. 1E and F); and the CSF cytology remained positive. Therefore, we treated the parenchymal recurrent mass with salvage TMZ chemotherapy (200 mg/m2 for 5 days every 4 weeks). After three cycles of TMZ treatment, MRIs of the brain and spine showed near complete responses of the previously enhanced lesions around the ventricle and spinal cord (Fig. 1G and H). In addition, CSF cytology revealed negative conversion. After six cycles of TMZ, T2 high signal intensity of multiple lesions on the cerebellum had improved (Fig. 1I). Following twelve cycles of TMZ, the patient was doing well and the Karnofsky performance score (KPS) score was 90. TMZ treatment continued without complications after 25 cycles. However, TMZ treatment was ceased because a follow-up MRI showed multiple cerebellar lesions without corresponding symptoms. Progression-free survival (PFS) was 25 months. Despite treatment of the cerebellar lesions with whole brain radiation with boost (6,000 cGy/25 fractions), the tumors progressed to the whole neuraxis, and the patient expired 14 months after the recurrence.
A 38-year-old woman presented with diplopia, headache, and nausea. The brain MRI revealed a T2 high signal intensity, ovoid mesencephalic mass lesion with spotty enhancement of the inferior portion (Fig. 3A and B). With a clinical diagnosis of a midbrain glioma, she received fractionated stereotactic radiotherapy (5,400 cGy/27 fractions). The MRI demonstrated a decrease in the extent of the lesion after 6 months radiation (Fig. 3C), and her symptoms were resolved. Three years after radiotherapy, a follow-up brain MRI revealed two small, enhancing nodules along the CSF pathways at the cerebellar tonsils and the lower posterior margin of the aqueduct (Fig. 3D). However, she only presented with intermittent headaches and diplopia, which were not specific for LMD. After 3 months, a follow-up MRI revealed slightly increased multiple enhancing nodules. At this time, the CSF cytology was positive for atypical cells, and the spinal MR showed diffuse spinal leptomeningeal seeding (Fig. 3E). The patient agreed to intraventricular chemotherapy, and, at the time of the Ommaya reservoir insertion, the ICP was elevated (29 cm H2O). Three cycles of VLP chemotherapy followed by weekly intrathecal methotrexate chemotherapy were completed. Six months after intrathecal chemotherapy, surveillance neuroimaging revealed multiple enhancing nodules involving the bifrontal white matter (Fig. 3F and G). The patient was subsequently administered 12 cycles of salvage TMZ chemotherapy (200 mg/m2 for 5 days every 4 weeks). Radiologic complete remission was obtained after six cycles simultaneously with cytologic remission (Fig. 3H). A solitary enhancing nodule at the cerebellopontine angle appeared after 12 cycles of TMZ chemotherapy (PFS: 12 months) (Fig. 3I). Resection, performed on September 13, 2013, revealed a WHO grade III anaplastic oligodendroglioma without 1p/19q chromosomal co-deletion (Fig. 4). The tumor was reclassified as a glioblastoma, IDH-wild type by the 2016 WHO new classification of gliomas in the same manner as Case 1. Although partial brain radiation was given to the residual tumor and the surrounding area (4,800 cGy/20 fractions), she developed progressive gait difficulty, a voiding problem, and cognitive dysfunction. Imaging revealed multiple progressions of seeding. A ventriculoperitoneal shunt was inserted to control the ICP, but she expired 6 months after completion of TMZ chemotherapy.
LMD from anaplastic gliomas is a rare manifestation. The incidence of LMD of anaplastic glioma is 6–21% [2]. Some autopsy studies revealed a higher incidence compared with clinical studies [89]. Symptomatic LMD occurs relatively late in the course of anaplastic gliomas and patients usually die before leptomeningeal progresses enough to provoke symptoms.
Saito et al. [2] reported that rates of intracranial and spinal dissemination from anaplastic astrocytomas and glioblastomas were 25% and 8.5%, respectively. Among 6 patients with spinal dissemination, one was an anaplastic astrocytoma and five were glioblastomas. Dardis et al. [10] reported 34 cases of LMD with grade III and IV gliomas, 24 with glioblastomas, and 10 with grade III tumors. According to a population-based study, LMD from anaplastic oligodendroglial tumors occurred in 2.9% of patients [11].
In contrast to LMD from other glioblastomas or systemic solid tumors, which tend to show a grave prognosis, LMD from anaplastic glial tumors shows a relatively favorable prognosis. In the series from Dardis et al. [10], patients with glioblastoma had a median overall survival of 9.9 months, while 18.9 months for the grade III gliomas. Saito et al. [2] reported that median survival times of patients with glioblastomas and anaplastic astrocytomas were 16 months and 40.5 months, respectively.
In senior patients and those with a high pathologic grade glioma, the time taken to the development of LMD appears to be shorter. Initial location in the temporal lobe and cerebellum seems to induce leptomeningeal spread more easily [10]. Some studies suggest that opening the ventricle may be a risk factor for LMD [12]. In our report, Case 1 was defined as a primary LMD, while Case 2 was diagnosed with LMD at the third recurrence. Primary diffuse leptomeningeal gliomatosis (PDLG) is an extremely rare manifestation characterized by leptomeningeal infiltration of glial malignant cells in the absence of primary parenchymal foci.
Given the rarity of anaplastic gliomatosis, the ability to develop an effective treatment is limited. Without large studies, management for anaplastic gliomatosis is currently guided by data acquired in the treatment of intracranial anaplastic gliomas. According to previous studies, treatment for LMD appears to improve outcomes. When the patient's KPS is over 70, maximal treatments are preferred as long as patients are tolerable.
Due to initial spinal LMD with simultaneous intracranial spread, Case 1 could not receive radiotherapy. Instead, systemic chemotherapy and VLP chemotherapy, which was especially effective in CSF flow impairment was applied. Despite successful chemotherapy, clinical and radiologic progression persisted. Against general expectation for his grave prognosis, the enhancing lesions around the whole neuraxis disappeared with cytologic negative conversion after 3 cycles of oral TMZ. Case 2 also showed meaningful outcome for recurrent cases after intrathecal chemotherapy for anaplastic gliomas. In recurrent cases, oral TMZ is considered an effective salvage therapy. In PDLG patients with malignant astrocytic cells reported by Hansen et al. [1], patients showed clinically valid outcomes with TMZ and radiation. Fanceschi et al. [13] and Jicha et al. [14] reported cases of primary LMD of anaplastic astrocytoma treated with TMZ. TMZ is characterized by good bioavailability, high central nervous system (CNS) penetration, and low rates of toxicity with resultant good tolerance. Upon administration of TMZ orally, CSF levels of TMZ reach approximately 20% of those measured in the plasma [4].
The recently revised WHO classification of tumors of the CNS defines tumor diagnosis with integrated phenotypic and genotypic parameters. The use of genotypic status, such as IDH mutation and 1p/19q co-deletion, results in an exclusive classification between astrocytomas and oligodendrogliomas. An anaplastic oligoastrocytoma, which was the diagnosis in Case 1, was discouraged in the 2016 CNS WHO classification. The lack of 1p/19q co-deletion is mismatched with the histological appearance [5]. According to revised histological diagnoses, both Case 1 and 2, which have no IDH mutation and 1p/19q co-deletion, were IDH-wild type glioblastomas.
These two patients, who showed positive CSF cytology, did not respond to chemoradiation, including ICE and intraventricular chemotherapy. TMZ was introduced when LMD presented with parenchymal lesion progression. Both patients experienced remarkable radiographic improvement, simultaneously with negative conversion of CSF cytology for a considerable period. To our knowledge, we present experience of oral TMZ to chemo-resistant anaplastic glioma patients.
Figures and Tables
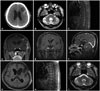 | Fig. 1Radiologic findings of Case 1. Initial axial T2-weighted magnetic resonance findings of a 54-year-old male patient diagnosed with anaplastic oligoastrocytoma (A and B), and sagittal and coronal T1-weighted images enhanced with gadolinium after the first craniotomy (C and D). Periventricular enhancement occurred after ifosphamide, carboplatin, and etoposide chemotherapy and intrathecal methotrexate (E and F). After treatment with temozolomide, leptomeningeal enhancement disappeared (G and H), and the T2-weighted signal intensity of the cerebellar lesion decreased (I). |
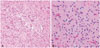 | Fig. 2Pathologic findings of Case 1. Increased cellularity and branching capillary networks resembling that of oligodendroglioma (A, original magnification, ×200; hematoxylin and eosin staining) and intermingled oligodendroglial cells having round nuclei and clear cytoplasm and astrocytic cells of elongated nuclei (B, original magnification, ×400; hematoxylin and eosin staining). |
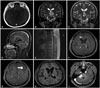 | Fig. 3Radiologic findings of Case 2. A second illustrative case of a 38-year-old female patient with a mesencephalic T2 high signal intensity lesion with spotty enhancement (A and B). After radiation, the extent of the lesion decreased (C), but metastasis developed 3 years later (D). Multiple enhancing nodules around the periventricular area occurred during ventriculolumbar perfusion chemotherapy (F and G). After six cycles of TMZ, imaging reveals resolution of the enhancing lesion (H), but another solitary lesion at the cerebellopontine angle appeared after 12 cycles of TMZ (I). TMZ, temozolomide. |
 | Fig. 4Pathologic findings of Case 2. High cellularity with closely packed polygonal cells of clear cytoplasm and branching capillary networks with tumor infiltration to surrounding granular layer of the cerebellum (A, original magnification, ×200; hematoxylin and eosin staining), and abundant clear cytoplasm and centrally located round to oval nuclei with nuclear pleomorphism (B, original magnification, ×400; hematoxylin and eosin staining). |
Acknowledgments
This work was supported by a grant (NCC1710871-1) from the National Cancer Center, Korea.
References
1. Hansen N, Wittig A, Hense J, Kastrup O, Gizewski ER, Van de. Long survival of primary diffuse leptomeningeal gliomatosis following radiotherapy and temozolomide: case report and literature review. Eur J Med Res. 2011; 16:415–419.

2. Saito R, Kumabe T, Jokura H, Shirane R, Yoshimoto T. Symptomatic spinal dissemination of malignant astrocytoma. J Neurooncol. 2003; 61:227–235.
3. Michotte A, Chaskis C, Sadones J, Veld PI, Neyns B. Primary leptomeningeal anaplastic oligodendroglioma with a 1p36-19q13 deletion: report of a unique case successfully treated with Temozolomide. J Neurol Sci. 2009; 287:267–270.

4. Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004; 10:3728–3736.

5. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131:803–820.

6. Sanson M, Ameri A, Monjour A, et al. Treatment of recurrent malignant supratentorial gliomas with ifosfamide, carboplatin and etoposide: a phase II study. Eur J Cancer. 1996; 32A:2229–2235.

7. Gwak HS, Joo J, Shin SH, et al. Ventriculolumbar perfusion chemotherapy with methotrexate for treating leptomeningeal carcinomatosis: a Phase II Study. Oncologist. 2014; 19:1044–1045.

8. Onda K, Tanaka R, Takahashi H, Takeda N, Ikuta F. Cerebral glioblastoma with cerebrospinal fluid dissemination: a clinicopathological study of 14 cases examined by complete autopsy. Neurosurgery. 1989; 25:533–540.

9. Yung WA, Horten BC, Shapiro WR. Meningeal gliomatosis: a review of 12 cases. Ann Neurol. 1980; 8:605–608.

10. Dardis C, Milton K, Ashby L, Shapiro W. Leptomeningeal metastases in high-grade adult glioma: development, diagnosis, management, and outcomes in a series of 34 patients. Front Neurol. 2014; 5:220.

11. Roldán G, Chan J, Eliasziw M, Cairncross JG, Forsyth PA. Leptomeningeal disease in oligodendroglial tumors: a population-based study. J Neurooncol. 2011; 104:811–815.

12. Bae JS, Yang SH, Yoon WS, Kang SG, Hong YK, Jeun SS. The clinical features of spinal leptomeningeal dissemination from malignant gliomas. J Korean Neurosurg Soc. 2011; 49:334–338.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download