Abstract
We report a rare case of subependymal giant cell astrocytoma (SEGA) associated with tumoral bleeding in a pediatric patient without tuberous sclerosis complex (TSC). A 10-year-old girl presented with a 2-week history of an increasingly aggravating headache. Brain magnetic resonance imaging revealed an approximately 3.6-cm, well-defined, heterogeneously enhancing mass with multistage hemorrhages on the right-sided foramen of Monro. The tumor was completely resected using a transcallosal approach. Intraoperatively, the mass presented as a gray-colored firm tumor associated with acute and subacute hemorrhages. The origin of the mass was identified as the ventricular septum adjacent to the foramen of Monro. A pathological analysis revealed pleomorphic multinucleated eosinophilic tumor cells with abundant cytoplasm. These cells showed positive staining for the glial fibrillary acidic protein and S100 protein. A diagnosis of SEGA was established. The patient recovered without any neurological symptoms. There was no evidence of TSC. The radiological follow-up showed no recurrence for 2 years. This was a case of SEGA with intratumoral hemorrhage, for which a favorable outcome was achieved, without any neurological deficit after tumoral resection.
Subependymal giant cell astrocytoma (SEGA) is a clinically benign tumor that is usually associated with tuberous sclerosis complex (TSC) [1]. TSC is an autosomal dominantly inherited neurocutaneous syndrome that affects any organ system of the body. The prevalence rate of TSC in patients with SEGA ranges from 5% to 20%. Solitary SEGAs in the absence of TSC-related lesions have been reported; these resulted from somatic mosaicism of the TSC gene or de novo mutations at the TSC locus [2345].
SEGA mostly occurs in the first two decades of life [67]. This tumor generally arises in the periventricular regions near the foramen of Monro. Therefore, diagnosis of SEGA is relatively easy, unless there is minimal evidence of tuberous sclerosis. Clinical features of SEGA include focal neurological deficits and symptoms related to increased intracranial pressure accompanying obstructive hydrocephalus. Occasionally, the tumor is associated with intratumoral bleeding [367]. Intratumoral bleeding causes acute neurological symptoms, and in some cases, the prognosis can be dismal. Here, we present a rare case of SEGA with intratumoral bleeding, without any signs of TSC.
A 10-year-old girl presented with a 2-week history of a continuous
headache, which was aggravated by accompanying nausea. Brain computed tomography showed acute hemorrhage in the region of the right lateral ventricle and obstructing hydrocephalus (Fig. 1A). Brain magnetic resonance image revealed an approximately 3.6-cm, well-defined mass on the body of the right lateral ventricle. The mass showed mixed low and high signal intensity on T1-weighted (Fig. 1B) and T2-weighted images (Fig. 1C). Following gadolinium administration, a heterogeneous enhancement was observed for the peripheral solid areas (Fig. 1D, E). The cerebral blood volume was focally increased in the MR-enhanced areas (Fig. 1F). The mass was a well-defined, heterogeneously enhanced lesion with mixed cystic and solid components. It contained multistage hemorrhages in the right-sided foramen of Monro. The radiological differential diagnoses included high-grade glioma, SEGA, and choroid plexus tumor. The tumor was completely resected via a transcallosal approach. Intraoperatively, the mass presented as a gray-colored, firm tumor associated with acute and subacute hemorrhages. The origin of the mass was the ventricular septum adjacent to the foramen of Monro. A pathological analysis revealed pleomorphic multinucleated eosinophilic tumor cells with abundant cytoplasm, associated with increased vascularity (Fig. 2A, B). The tumor cells showed focal positive staining for the glial fibrillary acidic protein (Fig. 2C) and strong positive staining for the S100 protein (Fig. 2D). The Ki-67 labeling index was less than 1%. A diagnosis of SEGA was established. The patient recovered without any neurological symptoms. On an evaluation of TSC, there is no family history of TSC and there were no cutaneous stigmata of tuberous sclerosis. A clinical evaluation was performed including echocardiography, renal ultrasonography, ophthalmologic examination, and skeletal imaging. No features of TSC were observed. A genetic study was not performed. The radiological follow-up showed no recurrence for 2 years (Fig. 1G, H).
SEGA is a rare tumor of the central nervous system with mixed glioneuronal features, most frequently seen in the setting of TSC [1]. The preoperative diagnosis of SEGA takes into account the age and clinical condition of the patient, and the location of the tumor. If clinical signs of TSC are present, an early diagnosis of TSC is possible. Solitary lesions without any clinical or radiographical evidence of tuberous sclerosis have been reported [234]. In these cases, the symptoms of SEGA are present with obstructing hydrocephalus, and in most cases, TSC is diagnosed according to the clinical diagnostic criteria, of which the presence of SEGA is the most important [8]. The tumor suppressor genes TSC1 on chromosome 9q34 and TSC2 on chromosome 16p13 encode the proteins tuberin and hamartin, respectively. In 20% of patients with clinically diagnosed TSC, mutations in the TSC1/2 genes are not observed, and the disease in patients without mutations is less severe than in those with TSC1/2 mutations [910]. It has been reported that solitary SEGAs that do not show any clinical evidence of TSC can be the result of somatic mosaicism [5]. Other reports have described solitary SEGAs with isolated somatic TSC2 mutations or amplification of exons on the TSC1 gene [211]. Solitary cases of SEGA without mutation may be due to epigenetic alteration in tuberin or hamartin [2]. The current case did not demonstrate any clinical features of TSC, and a genetic study was not performed.
Among primary brain tumors, the incidence of hemorrhage associated with glioma is 3–7%, with cases of low-grade glioma accounting for less than 1% [367]. Despite their slow-growing nature, tumoral hemorrhage has been reported with low-grade gliomas, and, in some cases, the prognosis has been dismal. The mechanisms of hemorrhage were investigated in malignant lesions [12], and include vascular structural abnormalities, which make the vessels fragile. When there is a change in blood volume or if tumor cells invade the vessels, tumoral hemorrhage or necrosis can easily occur in the fragile vascular structures. Due the relatively benign and slow-growing nature of low-grade gliomas, vascular proliferation and necrosis are not commonly present. Venous congestion, intravascular thrombosis, or vascular ectasia may represent potential mechanisms of hemorrhage [613]. A histological analysis of the current case revealed that the tumor contained many small vessels, which may have caused blood congestion in the tumor. Even though tumor bleeding is rare in low-grade glioma, the presentation of SEGA bleeding may be serious, and was found to be associated with significant morbidity and mortality in the reviewed cases. In three out of six cases of SEGA with tumoral hemorrhage, the patients had died or were in a vegetative state [6]. Perioperatively, one patient died as a result of venous hypothalamic infarction [14]. There is no doubt that doing prompt surgical removal in the acute increase in intracranial pressure due to obstructing hydrocephalus or hemorrhage [6].
For a benign SEGA, total surgical resection can be curative. Surgical treatment of symptomatic SEGA should be offered without debate [361314]. However, the appropriate time for surgery is still controversial for small asymptomatic lesions [151617]. Early surgical treatment for these lesions decreases the potential for surgical morbidity or hemorrhagic events compared with large lesions. However, the surgical treatment itself can cause morbidity, and tumoral hemorrhage is so rare that it may not always be considered. We found previous case reports of SEGA with intratumoral bleeding. As shown in Table 1, most of them underwent surgical treatment in the case of acute deterioration with intratumoral hemorrhage to reduce the mass effect to surrounding [7].
As alternative treatments, it can be considered Gamma Knife radiosurgery (GKR) or the mechanistic target of rapamycin (mTOR) inhibitors which can reduction the size of the mass in TSC-related tumors in not emergent situation like incidentally detected mass. GKR revealed good outcomes for many types of benign brain tumors, including gliomas, with a low incidence of side effects [22]. Out of reported cases of SEGA after GKR, half of them showed tumor volume reduction 70–80% within 6 months, and another shown tumor progression [22232425]. Even though the role of GKR in SEGAs was limited by the sporadic cases, these reported results suggest GKR may be an additional option for SEGAs that are small but progressively enlarging tumors where complete resection has not been safely achieved, residual or recurrent tumors. Recently, with encouraging preliminary results with rapamycin, a phase 2 open-label clinical trial using everolimus to treat SEGAs in 28 patients with TSC showed SEGA volume reduction of at least 30% in 21 patients (75%) and at least 50% in nine patients (32%) within the initial 6 months [26]. The therapy with everolimus was continued for a median duration of 21.5 months with trough concentrations of 5 to 15 ng/mL. Importantly, none of the patients treated with mTOR inhibitors required surgical intervention or developed new lesion during treatment [27]. However, the response has been shown to be temporary, lasting only as long as the medication is used, and the toxicity of the medication may exceed its benefits, due to necessity of long-term use of mTOR inhibitors. The tumor can regrow with the discontinuation of therapy, but it can also regrow with the continuation of therapy [2628].
In conclusion, hemorrhage is a rare occurrence in benign SEGAs; and, the outcome in such cases may be dismal. We presented an uncommon case of SEGA with intratumoral bleeding, without any signs of TSC. The patient recovered well following prompt surgical resection.
Figures and Tables
Fig. 1
Preoperative and postoperative radiological findings of subependymal giant cell astrocytoma with bleeding. A: A brain computed tomography scan showing acute hemorrhage on the right lateral ventricle. B: A brain magnetic resonance (MR) image showing an approximately 3.6-cm, well-defined mass in the right-sided foramen of Monro. The mass shows mixed low and high signal intensity on T1-weighted MR images. C: The mass shows mixed low and high signal intensity on T2-weighted MR images. D: Following gadolinium administration, the lesion demonstrates heterogeneous enhancement on axial images. E: The lesion is heterogeneously enhanced on peripheral solid area on sagittal images. F: Cerebral blood volume is focally increased on perfusion MR images. G and H: Recurrence is not observed for 2 years, based on T1-weighted enhanced MR images.
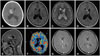
Fig. 2
Pathological findings of subependymal giant cell astrocytoma. A: Pathological analysis revealing tumor cells with many small vessels (hematoxylin and eosin staining, original magnification, ×40). B: The pleomorphic multinucleated eosinophilic tumor cells contain abundant cytoplasm (hematoxylin and eosin staining, original magnification, ×200). C: The tumor cells show focally positive staining for glial fibrillary acidic protein (original magnification, ×200). D: The tumor cells show positive staining for S100 protein (original magnification, ×200).
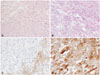
Table 1
Summary of published cases of SEGA with intratumoral bleeding

| Authors (years) | Patients age (years) | Signs & symptoms | Treatment | Recurrence | Outcome | F/U period |
|---|---|---|---|---|---|---|
| Waga et al. (1977) [18] | 11 | Hemiparesis | GTR | NA | Vegetative | NA |
| Barbosa-Coutinho et al. (1991) [19] | 13 | Headache, nausea | Tumor resection & HA | NA | Death | NA |
| Comatose M/S | ||||||
| Kalina et al. (1995) [20] | 17 | Seizure, lethargy | Tumor resection | NA | NA | NA |
| Hamamoto et al. (1994) [13] | 19 | Headache | External ventricular drainage | NA | Death | NA |
| Comatose M/S | ||||||
| Sinson et al. (1994) [14] | 21 | Headache, lethargy | Mass debulking | NA | Death | NA |
| Kim et al. (2001) [21] | 9 | Headache | NTR | None | Stable | 14 month |
| Stavrinou et al. (2008) [3] | 33 | Headache | GTR | NA | Stable | NA |
| Ogiwara and Morota (2013) [7] | 5 | Headache, vomiting | GTR | None | Stable | 6 month |
| Ogiwara and Morota (2013) [7] | 21 | Hemiparesis, vomiting lethargy | STR | None | Stable | 3 years |
| This case | 10 | Headache, nausea | GTR | None | Stable | 2 years |
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, & Future Planning (2014R1A1A1004469).
References
1. Adriaensen ME, Schaefer-Prokop CM, Stijnen T, Duyndam DA, Zonnenberg BA, Prokop M. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol. 2009; 16:691–696.

2. Beaumont TL, Godzik J, Dahiya S, Smyth MD. Subependymal giant cell astrocytoma in the absence of tuberous sclerosis complex: case report. J Neurosurg Pediatr. 2015; 16:134–137.

3. Stavrinou P, Spiliotopoulos A, Patsalas I, et al. Subependymal giant cell astrocytoma with intratumoral hemorrhage in the absence of tuberous sclerosis. J Clin Neurosci. 2008; 15:704–706.

4. Jung TY, Kim YH, Jung S, Baek HJ, Lee KH. The clinical characteristics of subependymal giant cell astrocytoma: five cases. Brain Tumor Res Treat. 2015; 3:44–47.

5. Kwiatkowska J, Wigowska-Sowinska J, Napierala D, Slomski R, Kwiatkowski DJ. Mosaicism in tuberous sclerosis as a potential cause of the failure of molecular diagnosis. N Engl J Med. 1999; 340:703–707.

6. Sterman H, Furlan AB, Matushita H, Teixeira MJ. Subependymal giant cell astrocytoma associated with tuberous sclerosis presenting with intratumoral bleeding. Case report and review of literature. Childs Nerv Syst. 2013; 29:335–339.

7. Ogiwara H, Morota N. Subependymal giant cell astrocytoma with intratumoral hemorrhage. J Neurosurg Pediatr. 2013; 11:469–472.

8. Northrup H, Krueger DA. International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013; 49:243–254.
9. Au KS, Williams AT, Gambello MJ, Northrup H. Molecular genetic basis of tuberous sclerosis complex: from bench to bedside. J Child Neurol. 2004; 19:699–709.

10. Dabora SL, Jozwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001; 68:64–80.

11. Ichikawa T, Wakisaka A, Daido S, et al. A case of solitary subependymal giant cell astrocytoma: two somatic hits of TSC2 in the tumor, without evidence of somatic mosaicism. J Mol Diagn. 2005; 7:544–549.
12. Wakai S, Yamakawa K, Manaka S, Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery. 1982; 10:437–444.
13. Hamamoto O, Honorato DC, Brito HL, Souza-Queiróz L. [Intratumor hemorrhage in tuberous sclerosis. A case report]. Arq Neuropsiquiatr. 1994; 52:435–438.
14. Sinson G, Sutton LN, Yachnis AT, Duhaime AC, Schut L. Subependymal giant cell astrocytomas in children. Pediatr Neurosurg. 1994; 20:233–239.

15. Beaumont TL, Limbrick DD, Smyth MD. Advances in the management of subependymal giant cell astrocytoma. Childs Nerv Syst. 2012; 28:963–968.

16. de Ribaupierre S, Dorfmüller G, Bulteau C, et al. Subependymal giant-cell astrocytomas in pediatric tuberous sclerosis disease: when should we operate? Neurosurgery. 2007; 60:83–89. discussion 89-90.
17. Fujiwara S, Takaki T, Hikita T, Nishio S. Subependymal giant-cell astrocytoma associated with tuberous sclerosis. Do subependymal nodules grow? Childs Nerv Syst. 1989; 5:43–44.
18. Waga S, Yamamoto Y, Kojima T, Sakakura M. Massive hemorrhage in tumor of tuberous sclerosis. Surg Neurol. 1977; 8:99–101.
19. Barbosa-Coutinho LM, Lima EL, Gadret RO, Ferreira NP. [Massive intratumor hemorrhage in tuberous sclerosis. Autopsy study of a case]. Arq Neuropsiquiatr. 1991; 49:465–470.
20. Kalina P, Drehobl KE, Greenberg RW, Black KS, Hyman RA. Hemorrhagic subependymal giant cell astrocytoma. Pediatr Radiol. 1995; 25:66–67.

21. Kim SK, Wang KC, Cho BK, et al. Biological behavior and tumorigenesis of subependymal giant cell astrocytomas. J Neurooncol. 2001; 52:217–225.
22. Henderson MA, Fakiris AJ, Timmerman RD, Worth RM, Lo SS, Witt TC. Gamma knife stereotactic radiosurgery for low-grade astrocytomas. Stereotact Funct Neurosurg. 2009; 87:161–167.

23. Park YG, Kim EY, Chang JW, Chung SS. Volume changes following gamma knife radiosurgery of intracranial tumors. Surg Neurol. 1997; 48:488–493.

24. Wang LW, Shiau CY, Chung WY, et al. Gamma knife surgery for low-grade astrocytomas: evaluation of long-term outcome based on a 10-year experience. J Neurosurg. 2006; 105 Suppl:127–132.

25. Park KJ, Kano H, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Gamma knife surgery for subependymal giant cell astrocytomas. Clinical article. J Neurosurg. 2011; 114:808–813.
26. Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010; 363:1801–1811.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download