Abstract
Over the course of five years, a total of ten cases were collected of glioma patients in whom a distant lesion at the fourth ventricle was noted. A ‘distant lesion’ was defined as a lesion with a normal appearing tissue bridge at imaging between the primary and secondary locations. Previous imaging of these patients was reviewed along with clinical history, course of therapy, and available histology. A review of the literature was performed with respect to present knowledge on patterns of glioma proliferation and dissemination. This case series is the first to describe the fourth ventricle as a location that may be prone to secondary lesions in glioma patients. Further investigation on this subject may yield deeper insights into the mechanisms by which glial tumors spread within the brain, with the hope of developing or improving therapeutic targets.
Gliomas are the most common primary cerebral malignancy and frequently carry a dismal prognosis. Therapy is often unsuccessful due to the tremendous variation in glioma behavior and diverse patterns of proliferation, which may reflect distinct molecular and genetic subtypes (an understanding reflected in the revised 2016 classification from the World Health Organization (WHO) [1]. In other malignancies of the human body, there are well-recognized patterns of metastasis through hematogenous or lymphatogenous dissemination of tumor cells. However, the concept of metastatic glioma has been scarcely discussed, amounting to a handful of case reports [234]. A metastatic or distant lesion refers to the emergence of tumor in a new location that is not contiguous with the primary lesion. With respect to intracerebral metastasis of gliomas, a distant lesion would be separated from the primary lesion by a bridge of normal-appearing parenchymal tissue and would develop chronologically subsequent to the primary lesion. Descriptions of intracerebral metastasis of gliomas are almost entirely absent in the medical literature. No information is available on which regions of the brain are particularly prone to developing metastatic lesions, nor is the pathophysiological mechanism of dissemination known.
The current study presents 10 cases of glioma in which a metastatic lesion was identified at the fourth ventricle border on magnetic resonance imaging (MRI). These lesions were distant from the primary lesion, separated by normal-appearing cerebral parenchyma. Below is an analysis of the features of these cases, followed by a discussion of possible pathophysiological mechanisms and clinical relevance.
A total of 10 glioma patients were identified who had developed a secondary lesion bordering the fourth ventricle. Four of these patients were identified in Winnipeg, Canada while six were identified in Germany.
Characteristics of the 10 patients are summarized in Table 1. The age range was from 36 to 61 years old, with a median of 50 years old. There were 2 females and 8 males. The pathology of the supratentorial tumors was either glioblastoma multiforme (GBM), grade II or grade III astrocytoma, as well as one initial grade II oligoastrocytoma. In addition, one of the grade III astrocytoma patients (patient #6) developed a recurrence 8 years later that was diagnosed as grade II oligoastrocytoma [5]. Biopsy of the secondary lesion was available in one German case (patient #7), confirming a grade II astrocytoma. Cerebrospinal fluid (CSF) cytology was not available in any of the cases.
The time interval preceding development of the fourth ventricular lesion was variable, with the lengthiest time being 15 years after the initial diagnosis. However, grade III astrocytomas and grade IV GBMs appeared to develop the secondary lesion sooner (within months to just over 3 years), whereas those patients with histologically low grade gliomas generally developed the secondary lesion between 6 to 15 years later. In the case of one 50-year-old male with a large left frontal glioma, the fourth ventricular tumor was already present at the time of the diagnosis with subsequent enlargement on follow-up (Fig. 1). Although a chronological sequence was not present in this patient to definitively characterize one lesion as primary or secondary, the size discrepancy between the lesions and the morphological resemblance to the other cases suggested a similar pattern of dissemination, and justified inclusion in this case series.
The location of the primary tumor was in the frontal or temporal lobes for all of the cases. The primary tumor abutted the lateral ventricle in only some instances. None of the patients demonstrated leptomeningeal spread of disease at MRI. There were no spinal or extracranial metastases in any of the selected cases. The only distant lesion established in these cases was the fourth ventricular tumor.
Most of the patients underwent surgical resection for their primary tumor prior to the appearance of the secondary lesion, with the exception of the patient who had the fourth ventricular lesion at initial presentation. Most of the patients received only radiation therapy and no chemotherapy. Patient #2 (Fig. 2) and patient #10 (Fig. 3) were diagnosed with grade III astrocytoma and received 12 cycles of temozolomide, while patient #1 with grade IV GBM declined chemotherapy (Fig. 4).
The distant lesion was identified by abnormal T2/fluid attenuation inversion recovery signal intensity in the cerebellum or cerebellar vermis adjacent to the fourth ventricle (Fig. 5). In many of the cases, the secondary lesion demonstrated gadolinium enhancement (Fig. 2E and 3H). Where spectroscopy was available, a characteristic elevated Cho: N-acetylaspartate ratio was identified. Progressive enlargement of the secondary lesion could progress to obstructive hydrocephalus (Fig. 2F). In patient 10 with grade III left frontal anaplastic astrocytoma, recurrent disease manifested with a fourth ventricular enhancing lesion accompanied with diffuse subependymal enhancement along the lateral ventricles (Fig. 3E-H); none of the other cases demonstrated subependymal enhancement.
Primary glial tumors are the most frequent intracranial neoplasms with approximately half of all primary brain tumors arising from glial cells. Of the glial tumors, GBM is the most frequent, representing approximately 50–60% of astrocytomas [6]. The prognosis of GBM is dismal, with a survival rate less than one year despite current therapeutic advances.
All patients diagnosed with GBM will eventually present with a tumoral recurrence after treatment. The majority (80–90%) will recur within 2 cm of the original tumor border. In rare cases, the tumor recurs rather distally from the original site. The mechanism underlying this pattern of tumoral spread is however not understood and presently subject to research.
High grade glial tumor can spread to the CSF giving the potential of spinal dissemination. The rate of spinal metastases can be variable from 0.4 to 2% reported in the literature. The infiltration of the CSF by GBM is approximately 15–25% in the autopsy studies [7].
The incidence of metastases outside the central nervous system is rare, with spread described to the bone marrow, liver, lung and lymph nodes. However, the incidence of metastatic spread is progressively increasing as the overall survival rate of these patients increases with improved therapies.
There are no prior publications identifying the particular pattern of dissemination seen in the present case series. These patients had different initial WHO grades of glial tumors, in different initial locations within the supratentorial brain. They were treated with different modalities, but they all presented with this unusual pattern of spread with a secondary tumor adjacent to the fourth ventricle, in the cerebellar hemisphere or cerebellar vermis. The time span between the initial diagnosis and the appearance of the secondary tumor also varied greatly between patients. The occurrence of secondary lesions at the fourth ventricle warrants dedicated investigation to ascertain the underlying mechanism of disease spread, which will help advance knowledge of glioma progression.
Glioma dissemination could conceivably occur by means of subependymal spread via the lateral ventricles, subarachnoid seeding, direct infiltration along white matter tracts, hematogenous spread, or dissemination through the recently described ‘glymphatic’ system. Subependymal spread is frequently manifested through diffuse subependymal enhancement, as see in one of the cases, although it may not be visualized at conventional MRI [8].
Infiltration along white matter tracts is the most common route of glial tumor spread. Dense white matter tracts, as found in the corpus callosum, the anterior commissure or the corticospinal tracts, offer tumors a predicted linear vector of dissemination. However, it is uncertain as to how infiltration along white matter tracts would result in fourth ventricular lesions, as a pattern of spread. Likewise, subarachnoid seeding explains leptomeningeal spread and ‘drop’ metastasis to the spine, but would not explain the fourth ventricle as a reproducible location of disease spread.
The presence of lesions adjacent to the fourth ventricle suggests a possible role for CSF dynamics in the spread of glial tumors. Other very recent preliminary case reports have also supported this hypothesis, given that recurrent distal lesions are noted adjacent to ventricles, even if the primary tumor was remote from the ventricular margin [9]. CSF flow dynamics may account for accumulation of tumor cells in the fourth ventricle, or may create an optimal environment for implantation in this region. Subependymal spread of tumor may be related to the cellular composition of the ‘subependymal zone’. During neurological development, there is intense cellular proliferation and long-distance migration from the subependymal zone. During adulthood, this layer may contain a population of quiescent stem cells with migratory potential, which may be uniquely susceptible to tumor infiltration or malignant transformation [1011].
There has been considerable debate surrounding the clearance of interstitial fluid from the brain parenchyma, particularly following recent proposals on the brain's lymphatic system-the ‘glymphatic’ network of perivascular CSF channels, as well as the meningeal lymphatic channels along the dural sinuses [1213]. The glymphatic system owes its name to the astrocytic glial cells responsible for producing a convection flow that draws in CSF along para-arterial channels, allowing clearance of solutes from the parenchymal interstitial fluid in a fashion similar to a lymphatic network [14]. The meningeal lymphatics represent an additional recently described system which has been hypothesized as a second step in the drainage of cerebral interstitial fluid following its drainage into the CSF via the glymphatic system [13].
While there are diverse views and numerous unanswered questions concerning the normal physiology of these drainage systems, there is even less knowledge on how these clearance pathways are altered in the presence of a neoplastic process. Neoplastic edema in the brain assuredly must have an impact on the convection and diffusion of interstitial fluid, and whether or not this plays a role in the dissemination of tumor into the CSF is an idea worth investigating.
In conclusion, the fourth ventricular predilection of secondary tumors has not been described prior to the current case series. Further research will be necessary to better understand the reasons for the occurrence of these secondary tumors in this particular location.
Figures and Tables
Fig. 1
50-year-old male (patient #3) presented to emergency with ataxia and diplopia. Initial MRI examination reveals a T2 hyperintense lesion in the left front lobe with extension of abnormal signal intensity across midline (A) and enhancement on T1-weighted post-gadolinium images (B). Evaluation of the posterior fossa demonstrates a T2 hyperintense (C) enhancing (D) lesion involving the cerebellar vermis at the fourth ventricle, present at the time of initial examination. MRI, magnetic resonance imaging.
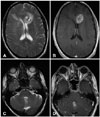
Fig. 2
53-year-old male with WHO grade III astrocytoma (patient #2). Initial MRI shows large right fronto-temporal FLAIR hyperintense (A) contrast-enhancing (B) lesion. Follow-up MRI obtained 3 years later reveals new abnormal FLAIR hyperintesity adjacent to the fourth ventricle (C). This lesion subsequently progressed with enlargement (D) and new enhancement (E), and eventually resulted in obstructive hydrocephalus (F). WHO, World Health Organization; MRI, magnetic resonance imaging; FLAIR, fluid attenuation inversion recovery.
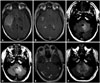
Fig. 3
50-year-old male with WHO grade III astrocytoma (patient #10). Initial MRI examination demonstrates a FLAIR hyperintense (A) contrast-enhancing (B) lesion in the parasagittal posterior left frontal lobe. Follow-up examination after two years reveals no recurrent tumor at the resection site on FLAIR (C) or T1-weighted post-gadolinium images (D). However, at 26 months, there are new areas of subependymal FLAIR hyperintensity (E) and enhancement (F) in the lateral ventricles. Furthermore, there is a discrete focus of abnormal FLAIR hyperintensity (G) and enhancement (H) involving the posterior left pons, abutting the fourth ventricle. WHO, World Health Organization; MRI, magnetic resonance imaging; FLAIR, fluid attenuation inversion recovery.
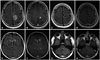
Fig. 4
36-year-old male (patient #1) with WHO grade IV astrocytoma (glioblastoma multiforme). Initial MRI examination reveals a FLAIR hyperintense (A) contrast-enhancing (B) lesion in the right frontal lobe. Follow-up MRI examination demonstrated a FLAIR hyperintense (C) non-enhancing (D) lesion abutting the fourth ventricle which developed 35 months after the initial lesion. WHO, World Health Organization; MRI, magnetic resonance imaging; FLAIR, fluid attenuation inversion recovery.

Fig. 5
39-year-old male with WHO grade II astrocytoma. Initial MRI reveals a large lesion in the left temporal lobe demonstrating abnormal FLAIR hyperintensity (A). After two years, the patient develops a new left cerebellar FLAIR-hyperintense lesion (B), histologically grade II astrocytoma. This lesion progressively enlarged as seen on a subsequent examination (C). WHO, World Health Organization; MRI, magnetic resonance imaging; FLAIR, fluid attenuation inversion recovery.
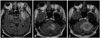
Table 1
Summary of characteristics of patients with secondary lesions

References
1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. Revised 4th ed. Lyon: International Agency for Research on Cancer;2016. p. 10–122.
2. Jezewski D, Parafiniuk D, Nowacki P, Kojder I. Intracerebral metastasis of glioblastoma multiforme. Case report and literature review. Ann Acad Med Stetin. 2011; 57:59–63. discussion 63-4.
3. Satter MR, Henry PT, Khan AI, Chowdhury Q, Hossain M, Kundu RK. Supratentorial glioblastoma multiforme metastasizing to the cervical spinal cord. Mymensingh Med J. 2014; 23:806–810.
4. Shahideh M, Fallah A, Munoz DG, Loch Macdonald R. Systematic review of primary intracranial glioblastoma multiforme with symptomatic spinal metastases, with two illustrative patients. J Clin Neurosci. 2012; 19:1080–1086.

5. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. 4th ed. Lyon: International Agency for Research on Cancer;2007. p. 8–93.
6. Hygino da Cruz LC Jr, Kimura M. Neuroimaging and genetic influence in treating brain neoplasms. Neuroimaging Clin N Am. 2015; 25:121–140.

7. Lawton CD, Nagasawa DT, Yang I, Fessler RG, Smith ZA. Leptomeningeal spinal metastases from glioblastoma multiforme: treatment and management of an uncommon manifestation of disease. J Neurosurg Spine. 2012; 17:438–448.

8. Cage TA, Pekmezci M, Prados M, Berger MS. Subependymal spread of recurrent glioblastoma detected with the intraoperative use of 5-aminolevulinic acid: case report. J Neurosurg. 2013; 118:1220–1223.

9. Goryaynov SA, Potapov AA, Ignatenko MA, et al. [Glioblastoma metastases: a literature review and a description of six clinical observations]. Zh Vopr Neirokhir Im N N Burdenko. 2015; 79:33–43.

10. Peretto P, Merighi A, Fasolo A, Bonfanti L. The subependymal layer in rodents: a site of structural plasticity and cell migration in the adult mammalian brain. Brain Res Bull. 1999; 49:221–243.

11. Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007; 9:424–429.

12. Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014; 11:26.

13. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015; 523:337–341.

14. Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013; 340:1529–1530.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download