Abstract
Astroblastoma is an uncommon glial tumor with predominant manifestation in the young age. Herein, we report a case of 18-year-old astroblastoma female patient who presented with history of two months headache. Magnetic resonance imaging (MRI) of the brain demonstrated well circumscribed, intra-axial abnormal signal intensity lesion (size=5×4 cm2) in the right parieto-occipital region of the brain. The patient underwent complete surgical resection of the gross tumor, as confirmed by an early post-surgical MRI (i.e., within 24 hours of surgery). Histopathological examination revealed neoplastic lesion exhibiting perivascular pseudo-rosettes with centrally hyalinized blood vessel and focal nuclear pleomorphism. Immunohistochemistry staining illustrated reactivity for glial fibrillary acidic protein and integrase interactor 1 (INI-1). These features rendered the diagnosis of astroblastoma. A comprehensive review of the current literature to summarize the clinicopathological and radiological characteristics, prognostic factors and current treatment strategies of astroblastomas is also presented. Our study would expand the pool of this uncommon tumor towards its better understanding and optimal treatment.
Astroblastoma is an uncommon brain tumor of glial origin, with typical manifestation in the young age, although few congenital cases have also been described [123]. In general, astroblastomas are large, peripheral and solid (with occasional cystic component) brain tumors. Typical clinical manifestation of these tumors includes headache, seizures, nausea, vomiting, and decay in conscious level [456]. The characteristic radiological appearance of astroblastoma more likely illustrate heterogeneous hyperintense signal on T2-wieghted sequences (T2WS), and fluid-attenuated inversion recovery (FLAIR) images, hypointense to isointense on T1-weighted sequences (T1WS), with characteristic “bubbly” appearance. The tumor show heterogeneous enhancement on contrast based T1WS images. The cystic component demonstrate rim enhancement. Tumor associated vasogenic edema may also be present [578910]. The histopathological features of astroblastoma include perivascular arrangement of neoplastic cells having thick, stout and short cytoplasmic processes with peripheral nuclei, forming pseudorosettes giving the characteristic “cartwheel” appearance [45611121314].
Herein, we report a case of 18-year-old female patient of astroblastoma, treated with complete surgical resection of the gross tumor. The study also present a comprehensive review of the current literature to summarize the clinical presentation, radiologic features, histology, immunohistochemistry, cytogenetics, prognosis, and management strategies of astroblastomas.
An 18-year-old female presented with a history of two months headache. No other clinical comorbidity was presented. Magnetic resonance imaging (MRI) of the brain demonstrated well circumscribed, intra-axial abnormal signal intensity lesion measuring 5×4 cm2 in the right parieto-occipital region of the brain. The lesion appeared heterogeneously hypointense to brain parenchyma on T1WS while heterogeneously hyperintense on T2WS and FLAIR, as shown in first row of Fig. 1. Post-contrast T1WS images showed heterogeneous contrast enhancing lesion with an eccentric non-enhancing necrotic core. Moreover, peri-lesional edema was also noted with minimal effacement of ipsilateral lateral ventricle (i.e., frontal horn and trigone). The patient underwent gross tumor resection (GTR) via right parieto-occipital craniotomy. Complete tumor resection was confirmed by an early post-surgical MRI (i.e., within 24 hours of surgery). Specifically, postsurgical MRI demonstrated abnormal signal intensity area in the tumor region, appearing predominantly hypointense on T1WS and hyperintense on T2WS and FLAIR imaging, signifying edema; however no definite residual mass was noted, as shown in second row of Fig. 1.
Histopathological examination revealed a neoplastic lesion with a fibrillary background. The neoplastic cells were columnar to elongated and showed abundant eosinophilic cytoplasm with a prominent, stout and tapering cellular process terminating on central hyalinized blood vessels in rosette like fashion (perivascular pseudo-rosettes). In few areas, the pseudo-rosettes showed central sclerosis resulting in a papillary configuration. Nuclei were round to oval. Focal nuclear pleomorphism along with extensive fibrous areas and few mitotic figures were seen. However, areas of necrosis and vascular proliferation were not identified. Immunohistochemical (IHC) staining illustrated the following pattern: positivity for integrase interactor 1 and diffuse positivity for glial fibrillary acidic protein (GFAP); negative pattern was observed for epithelial membrane antigen (EMA), cytokeratin CAM 5.2 and cytokeratin AE1/AE3. The proliferative index Ki-67 (Mib-1) was up to 5%. Illustrative hematoxylin and eosin and IHC images are shown in Fig. 2. These features were consistent with astroblastoma exhibiting low proliferation rate.
Afterwards, metastatic workup of the patient including x-ray chest and ultrasound abdomen and pelvis did not revealed any spread of the disease. Moreover, no significant lymphade-nopathy was present and history of any familial malignancy was negative. Hematology analyses did not revealed any abnormality. The clinical features, tumor characteristics and IHC analysis of the patient are summarized in Table 1. Adjuvant radiotherapy was not offered due to evidence of low-grade features (Ki-67≤5%). Presently, the patient is disease-free and has been kept on close follow up. The patient granted written consent for using/publishing his data in research/academic activities.
Astroblastoma is a rare tumor of glial origin, with predominant manifestation in young age. Herein, we have reported the first case, to the best of our knowledge, of astroblastoma from Pakistan, as part of our project entitled as “centralized registry program for uncommon tumors,” where incidence of several uncommon malignancies have been recently reported from this platform [151617]. This registry program would hopefully establish a reliable national database of uncommon tumors, not only expanding the pool of uncommon cancers for their better understanding but also enabling the comparison of our epidemic patterns with similar studies.
The clinical manifestations of astroblastoma tend to correlate with the raised intracranial pressure caused by the tumor, which in turn depend on the size, mass and localization of the tumor and may vary significantly among the individual patients. Typically, astroblastoma develop in the cerebral hemispheres, most likely affecting the frontal lobe followed by parietal and temporal lobes [2]. Nevertheless, such tumors has also been reported at other locations of the central nervous system, like the corpus callosum, cerebellum, brainstem, intraventricular or cauda equine [51819].
The most common clinical symptoms of astroblastoma include headache [6], as also observed in this study. History of seizures, nausea, vomiting, progressive hemiparesis and coma has also been reported [456]. Occasionally, symptoms such as diplopia, dizziness and confusion have also been documented [20]. Contrary to intracranial metastatic tumors (e.g., clear cell renal carcinoma, choriocarcinoma, melanoma, etc.) that have a tendency to bleed, brain hemorrhage appears a relatively uncommon (-5%) initial presentation in astroblastoma [22122]. Indeed, fresh hemorrhage (and central necrosis) was recognized in the cut surface of the resected astroblastoma tumor [6]. Tumialán et al. has also described hemorrhage in association with low-grade astroblastoma, which was mimicking a cavernous malformation on the imaging studies [421].
Various studies have described the gender-wise trends of astroblastoma incidence; while the Surveillance, Epidemiology, and End Results (SEER) data analysis reported almost equal incidence in male and female (51.1% vs. 48.9%) [3], majority of the studies reported a female predominance. Specifically, the male to female ratio of 1:3 [4], 1:2 [2], 1:2.4 [19], and 1:2.1 [23] has been reported.
The common radiological signatures of astroblastoma tumors can be potentially characterized using both MRI and computed tomography (CT) studies. In this context, such tumors tend to appear well circumscribed, hemispheric, often presenting a cystic mass component and generally located peripherally (i.e., near to or at the surface of the brain). Calcifications are often present, particularly with solid tumors. Moreover, these tumors mostly demonstrate contrast-based heterogeneous enhancement on both CT and MRI [823].
For MRI, the signal intensities of astroblastoma tumors differ for the T1WS, T2WS, and FLAIR images. Specifically, the tumor most likely appears hypointense in T1WS while hyperintense in T2WS and FLAIR images, with well-defined borders [824]. The solid component of the tumor often demonstrates a bubbly appearance, which could be easily appreciated on the FLAIR images. The cystic components, if present, demonstrate a characteristic rim enhancement [24]. Moreover, both the original and recurrent lesions usually have similar radiologic appearance [456]. Preoperative peritumoral edema most likely generate homogenously hyperintense signal on T2WS- and FLAIR images. Consequently, postoperative early MRI is usually beneficial to assess any residual tumor after surgery, which could otherwise be misinterpreted as recurrent tumor in later studies [5]. With gadolinium enhancement, the solid element show heterogeneous enhancement while the cystic component show ring enhancement [26]. The characteristic radiological features observed in our patient (i.e., well-circumscribed, heterogeneously enhancing peripheral tumor) were consistent with these studies.
Previously, characterization of astroblastoma tumors on cranial CT has been linked to radiological features such as isodense or slightly hyper-attenuated signal to the brain parenchyma and heterogeneous enhancement with contrast administration (particularly for the solid component of the tumor) [578910]. Calcifications are typically described as punctate on CT [711].
The characteristic imaging features and tumor location may also help to distinguish astroblastoma from meningioma and ependymoma. Specifically, unlike astroblastoma, meningioma express tendency towards homogeneous enhancement. In addition, astroblastoma typically arise in the supratentorial location, while ependymomas usually involve the posterior fossa. The calcifications found in astroblastoma may help distinguishing it from glioblastomas [9].
Several studies have documented the characteristic histopathological features of astroblastoma. In particular, the most common features of astroblastoma include perivascular pseudorosette pattern, foci of stromal hyalinization, increased cellularity with prominent nuclear pleomorphism, high proliferation rate and necrotic zones [45611]. Moreover, focally prominent pseudopapillary architecture, columnar to elongated neoplastic cells, occasional calcification and lack of fibrillarity have also been noted [11121314]. Alternatively, the distinguishing histological and ultra-structural features of ependymoma include well- developed junctional complexes, abundant microfilaments and clusters of microvilli [2526]. A significant overlap in histologic features of astroblastoma with glioblastoma has been demonstrated [27].
IHC analyses of astroblastoma typically demonstrate immunereactivity for GFAP, vimentin and S-100 protein [4]. These tumors also display occasional immunoreactivity for EMA. However, the tumor cells are usually negative for cytokeratins [4]. The proliferative index Ki-67 (Mib-1) of astroblastoma tumors may be high [456].
Some studies have also speculated on the characteristic cytogenetic profile of astroblastomas, which revealed that the most frequent chromosomal alterations were gains of chromosomes 19 and 20q, detected by comparative genomic hybridization. These alterations frequently occur together. Additionally, the combination of these two gains and losses on 9q, 10, and X have also been observed [14]. Recently, the presence of BRAFV600E mutations has been shown in a subset (n=21/28 cases) of astroblastomas [28]. Moreover, isocitrate dehydrogenase 1/2 (IDH1/2) mutations, common in gliomas, have been also suspected in astroblastoma [2429].
The optimal management strategy of astroblastoma tumors remains inconclusive, presumably due to the rarity of the disease; however, total surgical resection of the tumor seems the preferred and easier intervention due to the well demarcated nature and peripheral location of these tumors [41213]. In high grade astroblastoma patients, adjuvant radiotherapy to eradicate the tumor bed has been suggested [412]. Alternatively, the role of adjuvant chemotherapy remains unclear [412].
Recently, Mallick et al. [2] has reviewed and analyzed the patterns of survival outcomes in astroblastoma patients (n=152) managed with different treatment strategies; a lack of agreement to offer adjuvant treatment has been concluded. Specifically, it was found that the astroblastoma treatment option varies from surgery alone to surgery followed by adjuvant radiotherapy, surgery followed by adjuvant chemotherapy, or surgery followed by both adjuvant radio-and chemo-therapy. In particular, nearly half of the patients (i.e., 46%) were treated with surgery alone, 34% received surgery followed by adjuvant radiotherapy, and 18% received tri-modality therapy (i.e., surgery, radio- and chemo-therapy). Moreover, the patients treated with GTR were found to have better survival compared to those treated with sub-total resection (STR) of the tumor (p=0.0649) [2]. These survival trends are consistent with other similar studies. For instance, in a large cohort of patients (n=116), statistically significant (p=0.011) difference in 5-year progression-free survival has been observed for patients treated with GTR as compared to STR (83% vs. 55%) [19]. The SEER data (n=206) also revealed similar patterns of survival; patients treated with surgery alone compared to only radiation therapy showed improved 5-year overall survival (OS) (62.2% vs. 27.3%; p<0.001) and cause-specific survival (67.3% vs. 31.9%; p<0.003) [3]. Shen et al. [30] has also suggested GTR for low-grade patients, based on the National Comprehensive Cancer Network guidelines. Moreover, a 60-year-old female patient of astroblastoma treated with radical surgery alone remained without any evidence of tumor recurrence for a period of over two years [24]. In conclusion, the current literature pertaining to the treatment of astroblastoma indicates that GTR alone provides better survival outcome, particularly for low grade tumors.
The role of adjuvant radiotherapy to the tumor bed has been debatable. Adjuvant radiotherapy may not be necessary in low-grade astroblastoma tumors, particularly after radical surgery (i.e., GTR). Alternatively, focal radiotherapy has been suggested largely in patients with high-grade tumors, tumors with STR, or in the setting of tumor recurrence [3121431]. That said, an 8-year-old male patient of high-grade astroblastoma underwent radical surgery followed by radiotherapy to the tumor bed (dose=59.40 Gy) was disease-free at 54 months follow-up [6]. The definitive role of chemotherapy in the adjuvant setting is also not clear. For instance, a 4-year-old boy managed with multimodality treatment (i.e., surgery, followed by adjuvant radiotherapy, 54 Gy, and chemotherapy, including cisplatin and etoposide) remained disease-free at 8 months follow-up [13]. Another 50-year-old female patient treated with surgery followed by radiotherapy (dose=54 Gy) and chemotherapy remained free of disease for more than 6 years [32]. However, no definitive comment can be made on the basis of such scarce reports. Moreover, few studies have advocated the use of temozolomide, a chemotherapy agent frequently employed in the treatment of both low- and high-grade gliomas.
The prognosis of astroblastoma is not well understood. However, several factors of prognostic importance have been speculated. The extent of surgical resection seems to contribute significantly towards favorable prognosis [3514]. Interestingly, astroblastoma tumors typically express an expanding growth pattern compressing the surrounding normal tissue without infiltration, as opposed to infiltrative gliomas, thereby facilitating tumor resection [4]. Moreover, early post-surgical MRI may be helpful in evaluating the status of any residual tumor, which, in turn, could also provide a reference to assess tumor recurrence/progression in subsequent studies [5].
Importantly, prognosis of astroblastoma appears to directly correlate with its histological grade (i.e., favorable prognosis seen for tumors with low-grade histology compared to those with high-grade histology) [263334]. Specifically, low-grade tumors are characterized by their well-delineated shape, minimal proliferation index (Ki-67), no cellular atypia and no proliferation of vascular endothelium, as opposed to high-grade tumors that express higher proliferation rate, cellular atypia and hypertrophy of the vascular endothelium [26]. The extent of peritumoral T2WS signal on MRI and tumor necrosis may also help differentiating the tumor grade [9]. Interestingly, high-grade astroblastomas treated with GTR may offer relatively better prognosis, indicating that histological grade alone may not always be the best predictor of clinical outcome [31]. Tumor site has also been associated with clinical outcome [2346121333]. For instance, the 5-year OS for tumors located at infra- and supra-tentorial has been estimated at 75% and 44.9% (p<0.001), respectively [3]. Moreover, the preoperative peritumoral edema has been considered an unfavorable prognostic feature, which has been correlated with early recurrence or progression, particularly in low-grade astroblastomas. Specifically, comparison of recurrence rate in high- vs. low-grade astroblastomas with peritumoral edema has been demonstrated at 60% vs. 23.5% [935].
In addition to the degree of surgical resection and histological type, the age and gender of the patient also seems to be of prognostic significance [461213]. Typically, astroblastoma appears in young-adult patients. However, a strong correlation between age and OS- the worse OS correlated with older agehas been reported [23]. Furthermore, contrary to the SEER data which reported almost equal incidence of astroblastoma in male and female patients [3], majority of the studies have described a predominant incidence in female patients [241923].
In conclusion, we presented an uncommon case of astroblastoma, whose management was carried out with radical resection of the tumor through craniotomy, followed by close follow up without offering any other treatment. The clinical presentation, radiologic features, histology, IHC, cytogenetics, prognostic factors and management strategies of astroblastomas has been reviewed from the current literature. Given the rarity of the astroblastoma, this study may assist in providing a better understanding of the characteristic features and optimal treatment.
Figures and Tables
Fig. 1
MR imaging study of the brain. First row, pre-surgery MR images illustrating a well-circumscribed tumor in the right parieto-occipital region of the brain measuring 5×4 cm2; the lesion appeared heterogeneously hypointense to brain parenchyma on T1WS (A) while heterogeneously hyperintense on T2WS (B) and FLAIR (C). Second row, post-surgery MR images illustrating no definite residual mass and abnormal signal intensity area in tumor region, appearing predominantly hypointense on T1WS and hyperintense on T2WS and FLAIR signifying edema. MR, magnetic resonance; T1WS, T1 weighting sequence; T2WS, T2 weighting sequence; FLAIR, fluid-attenuated inversion recovery.
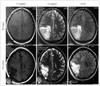
Fig. 2
Illustrative histopathology (hematoxylin and eosin) and immunohistochemical images of the resected astroblastoma specimen. A and B: Pseudo-papillary configuration at low magnification (×10). The arrow is pointing towards central blood vessel. C and D: arrow marking vascular hyalinization which is a conspicuous feature of astroblastomas and ranges from mild to severe (×20). E and F: tumor cells arranged in perivascular pseudo-rosette like fashion at higher magnification (×40). Arrow pointing towards central blood vessel wall, G: Ribbon like appearance of astroblastic rosettes on tangential section. It shows classic morphology of tumor displaying columnar to elongated tumor cells with tapering cytoplasmic process pointed by arrow at higher magnification (×40). H: Low proliferative index on Ki-67 (×10). I: glial fibrillary acidic protein immunostain positivity (×20).
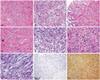
Table 1
Summary of clinical features of the patient with astroblastoma, tumor characteristics and status of immunochemical staining

References
1. Pizer BL, Moss T, Oakhill A, Webb D, Coakham HB. Congenital astroblastoma: an immunohistochemical study. Case report. J Neurosurg. 1995; 83:550–555.
2. Mallick S, Benson R, Venkatesulu B, Melgandi W, Rath GK. Patterns of care and survival outcomes in patients with astroblastoma: an individual patient data analysis of 152 cases. Childs Nerv Syst. 2017; 33:1295–1302.

3. Ahmed KA, Allen PK, Mahajan A, Brown PD, Ghia AJ. Astroblastomas: a Surveillance, Epidemiology, and End Results (SEER)-based patterns of care analysis. World Neurosurg. 2014; 82:e291–e297.

4. Johnson KA, Bonnin JM, Boaz JC, Douglas-Akinwande AC, Hattab EM. Anaplastic astroblastoma presenting as massive, sudden-onset, intraparenchymal hemorrhage. Pediatr Neurosurg. 2010; 46:457–461.

5. Miranda P, Lobato RD, Cabello A, Gómez PA, Martínez de. Complete surgical resection of high-grade astroblastoma with long time survival: case report and review of the literature. Neurocirugia (Astur). 2006; 17:60–63.

6. Mangano FT, Bradford AC, Mittler MA, Valderrama E, Schneider SJ. Astroblastoma. Case report, review of the literature, and analysis of treatment strategies. J Neurosurg Sci. 2007; 51:21–27. discussion 27.
7. Cunningham DA, Lowe LH, Shao L, Acosta NR. Neuroradiologic characteristics of astroblastoma and systematic review of the literature: 2 new cases and 125 cases reported in 59 publications. Pediatr Radiol. 2016; 46:1301–1308.

8. Baka JJ, Patel SC, Roebuck JR, Hearshen DO. Predominantly extraaxial astroblastoma: imaging and proton MR spectroscopy features. AJNR Am J Neuroradiol. 1993; 14:946–950.
9. Samples DC, Henry J, Bazan C, Tarasiewicz I. A case of astroblastoma: radiological and histopathological characteristics and a review of current treatment options. Surg Neurol Int. 2016; 7:Suppl 40. S1008–S1012.

10. Sugita Y, Terasaki M, Shigemori M, Morimatsu M, Honda E, Oshima Y. Astroblastoma with unusual signet-ring-like cell components: a case report and literature review. Neuropathology. 2002; 22:200–205.

11. Port JD, Brat DJ, Burger PC, Pomper MG. Astroblastoma: radiologic-pathologic correlation and distinction from ependymoma. AJNR Am J Neuroradiol. 2002; 23:243–247.
12. Salvati M, D'Elia A, Brogna C, et al. Cerebral astroblastoma: analysis of six cases and critical review of treatment options. J Neurooncol. 2009; 93:369–378.

13. Unal E, Koksal Y, Vajtai I, Toy H, Kocaogullar Y, Paksoy Y. Astroblastoma in a child. Childs Nerv Syst. 2008; 24:165–168.

14. Brat DJ, Hirose Y, Cohen KJ, Feuerstein BG, Burger PC. Astroblastoma: clinicopathologic features and chromosomal abnormalities defined by comparative genomic hybridization. Brain Pathol. 2000; 10:342–352.

15. Sadiq M, Ahmad I, Shuja J, Ahmad K. Primary Ewing sarcoma of the kidney: a case report and treatment review. CEN Case Rep. 2017; [Epub]. DOI: 10.1007/s13730-017-0259-0.

16. Shuja J, Ahmad I, Ahmad K, et al. Pleuropulmonary blastoma. J Cancer Res Pract. 2017; 4:111–114.

17. Iftikhar S, Ahmad I, Qasmi IM, Ahmad K, Manzoor H. Tuberous sclerosis complex with sub-ependymal giant cell astrocytomas; a case report. J Cancer Res Pract. 2017; [Epub]. DOI: 10.1016/j.jcrpr.2017.05.002.
18. Notarianni C, Akin M, Fowler M, Nanda A. Brainstem astroblastoma: a case report and review of the literature. Surg Neurol. 2008; 69:201–205.

19. Sughrue ME, Choi J, Rutkowski MJ, et al. Clinical features and postsurgical outcome of patients with astroblastoma. J Clin Neurosci. 2011; 18:750–754.

20. Bell JW, Osborn AG, Salzman KL, Blaser SI, Jones BV, Chin SS. Neuroradiologic characteristics of astroblastoma. Neuroradiology. 2007; 49:203–209.

21. Tumialán LM, Brat DJ, Fountain AJ, Barrow DL. An astroblastoma mimicking a cavernous malformation: case report. Neurosurgery. 2007; 60:E569–E570. discussion E570.
22. Bitoh S, Hasegawa H, Ohtsuki H, Obashi J, Fujiwara M, Sakurai M. Cerebral neoplasms initially presenting with massive intracerebral hemorrhage. Surg Neurol. 1984; 22:57–62.

23. Navarro R, Reitman AJ, de León GA, Goldman S, Marymont M, Tomita T. Astroblastoma in childhood: pathological and clinical analysis. Childs Nerv Syst. 2005; 21:211–220.

24. Fu YJ, Taniguchi Y, Takeuchi S, et al. Cerebral astroblastoma in an adult: an immunohistochemical, ultrastructural and genetic study. Neuropathology. 2013; 33:312–319.

25. Barakat MI, Ammar MG, Salama HM, Abuhashem S. Astroblastoma: case report and review of literature. Turk Neurosurg. 2016; 26:790–794.
26. Kubota T, Sato K, Arishima H, Takeuchi H, Kitai R, Nakagawa T. Astroblastoma: immunohistochemical and ultrastructural study of distinctive epithelial and probable tanycytic differentiation. Neuropathology. 2006; 26:72–81.

27. Bale TA, Abedalthagafi M, Bi WL, et al. Genomic characterization of recurrent high-grade astroblastoma. Cancer Genet. 2016; 209:321–330.

28. Lehman NL, Hattab EM, Mobley BC, et al. Morphological and molecular features of astroblastoma, including BRAFV600E mutations, suggest an ontological relationship to other cortical-based gliomas of children and young adults. Neuro Oncol. 2017; 19:31–42.

29. Kato Y. Specific monoclonal antibodies against IDH1/2 mutations as diagnostic tools for gliomas. Brain Tumor Pathol. 2015; 32:3–11.

30. Shen F, Chen LC, Yao Y, Zhou LF. Astroblastoma: rare incidence and challenges in the pattern of care. World Neurosurg. 2014; 82:e125–e127.

31. Bonnin JM, Rubinstein LJ. Astroblastomas: a pathological study of 23 tumors, with a postoperative follow-up in 13 patients. Neurosurgery. 1989; 25:6–13.

32. Bergkåsa M, Sundstrøm S, Gulati S, Torp SH. Astroblastoma-a case report of a rare neuroepithelial tumor with complete remission after chemotherapy. Clin Neuropathol. 2011; 30:301–306.

33. Yuzawa S, Nishihara H, Tanino M, et al. A case of cerebral astroblastoma with rhabdoid features: a cytological, histological, and immunohistochemical study. Brain Tumor Pathol. 2016; 33:63–70.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download