Abstract
A 19-year-old man presented with bitemporal hemianopsia and was found to have a large sellar and suprasellar tumor, resembling a pituitary macroadenoma. Emergency transsphenoidal approach was attempted because of rapid visual deterioration with headache. However, the approach was complicated and stopped by uncontrolled hemorrhage from the tumor. After conventional cerebral angiography and recognition of an unusual pathology, transcranial approach was achieved to prevent permanent visual loss. The final pathological diagnosis was pituicytoma with epithelioid features. Pituicytoma is a rare low-grade tumor (WHO Grade I) of pituicytes involving the sellar and suprasellar region, and originating from special glial cells of the neurohypophysis. Because of the high vascularity, the firm consistency, and invasion to surrounding neurovascular structures, a pituicytoma should be included in the differential diagnosis of a mass in the sellar and suprasellar area if the tumor shows high enhancement with vascular components. We report a case of rare pituicytoma mimicking a pituitary macroadenoma with massive hemorrhage to disturb surgery.
Pituicytoma is a rare benign sellar and suprasellar neoplasm originating from the neurohypophysis or the infundibulum. The tumor is classified as a distinct entity based on the World Health Organization (WHO) classification of central nervous system tumors in 2007 [1]. It is difficult to differentiate pituicytomas from other sellar and suprasellar neoplasms, such as pituitary adenomas, meningiomas, granular cell tumors, and pilocytic astrocytomas because of no specific radiological findings and low incidence. Therefore, they are not ordinarily included in the differential diagnosis of sellar and suprasellar tumors. The pituicytomas are histologically benign tumors, but their hypervascularity during surgery embarrasses surgeons because surgical removal is difficult. We review and evaluate the clinical and surgical features of pituicytomas and the relevant literatures.
A 19-year-old man presented to our department with progressive visual deterioration for 6 months. Ophthalmological examination revealed bitemporal hemianopsia and reduced visual acuity that was more apparent in his right eye (visual acuity: right eye, 0.2; left eye, 0.6). Hormonal evaluation revealed values within normal ranges except for hypogonadism [testosterone 0.04 ng/mL (normal, 2.67–10.12 ng/mL)]. Brain magnetic resonance imaging (MRI) showed a 3.8×3.1×4.5 cm sized highly enhancing mass expanding the sella and extending into the suprasellar cistern, compressing the optic chiasm, and also displacing A1 segments of the anterior cerebral arteries superiorly and anteriorly, suggestive of a pituitary macroadenoma. The tumor was hypointense on T1-weighted images and hyperintense on T2-weighted images. Intense and homogenous enhancement are presented (Fig. 1). Based on the clinical and radiological features, the tumor was assumed to be a pituitary macroadenoma with optic neuropathy, and transsphenoidal approach (TSA) was initially scheduled for elective surgery. However, we performed an emergency TSA because his vision rapidly deteriorated during preoperative systemic evaluation for elective surgery. TSA surgery revealed that the tumor had a rubbery-firm consistency, hypervascularity, and massive bleeding. Because of unexpected severe bleeding that needed several units of blood transfusion and confusing pathology, surgery was prematurely stopped after only a partial resection for pathological diagnosis and application of several hemostatic agents to control bleeding.
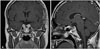
We planned a second stage transcranial approach for decompression of the optic nerves and chiasm after digital subtraction cerebral angiography. The cerebral angiogram showed multiple feeders from both internal carotid arteries. The tumor blush was noted in the mid- and late-arterial phases (Fig. 2). We could not perform a tumor embolization because of multiple small feeders from both internal carotid arteries. Consecutively, the tumor was approached through a right fronto-temporal craniotomy to prevent permanent visual loss. At surgery, the tumor was soft, highly vascular, and appeared to be originating from the pituitary stalk. We achieved gross total resection of the suprasellar mass to reduce compression of the optic apparatus. The pituitary stalk could not be preserved due to intrinsic adhesion of the tumor. On the other hand, we removed the intrasellar mass subtotally because of uncontrolled bleeding from the remnant attached to both cavernous sinuses. Subsequent pathology revealed a pituicytoma with epithelioid features. Microscopic examination showed a hypercellular tumor, composed of predominantly epithelioid cells with a vague storiform and perivascular architecture. The epithelioid tumor cells had pale eosinophilic cytoplasm and ovoid nuclei with small nucleoli. The tumor cells were positive for thyroid transcription factor (TTF)-1 and S-100 protein, and were focally positive for glial fibrillary acidic protein (GFAP), which suggested the glial nature of the tumor cells. Positive immunostaining for TTF-1 and morphologic features support the diagnosis of a pituicytoma with epithelioid features (Fig. 3). Postoperatively, the patient developed panhypopituitarism and diabetes insipidus. Levothyroxine, hydrocortisone, and DDAVP were given. In the postoperative period, the patient's visual acuity and field defect improved. A postoperative MRI with gadolinium enhancement showed the residual tumor adherent to both cavernous sinuses (Fig. 4). We decided to manage the residual tumor with gamma knife radiosurgery. The follow-up magnetic resonance scans showed the residual tumor to be stable.
In 1958, Liss and Kahn [2] described a tumor arising from the posterior part of the pituitary gland as the term “pituicytoma”. In 2000, Brat et al. [3] presented specific pathological distinction for the diagnosis of pituicytomas. Pituicytoma was defined as low-grade, spindle cell astrocytic tumors that originates in the posterior pituitary or its stalk based on the 2007 WHO classification of CNS tumors [1]. In the current 2016 WHO classification of CNS tumors, the term pituicytoma was restricted to a distinct group of low-grade glial tumors found in the posterior pituitary and infundibulum, presumably arising from pituicytes. Basically, the neurohypophysis is composed of the posterior pituitary gland, pituitary stalk, infundibulum, and median eminence. The cellular elements are microglia, pituicytes, and the distal nerve cells from anastomosed blood vessels and the hypothalamus. Pituicytes are considered to be modified neuroglial cells and positive immunohistochemical staining for GFAP. TTF-1 is strongly expressed in fetal and adult human pituicytes. It is also expressed in the normal development of the ventral forebrain and pituitary gland. Lee et al. [4] reported that TTF-1 is specifically expressed in pituicytomas, granular cell tumors, and spindle cell oncocytomas, and is also useful for distinguishing them from other sellar tumors. Primary tumors that arise in the neurohypophysis are rare, and are reported as many different diagnosis such as pituicytomas, infundibulomas, choristomas, and granular cell tumors [5]. It appears to have a slight predominance in men (59%) and occurs most often during the middle decades (mean age, 50 years old) [6]. However, the epidemiology is not well known because of low incidence of the tumor.
The clinical symptoms of pituicytomas are variable according to tumor size and location. The endocrine symptoms of a pituicytoma in the sella are not different from a typical pituitary adenoma, presenting sexual dysfunction that is the most common endocrinopathy in sellar tumors. On the other hand, suprasellar tumors usually present with visual symptoms caused by direct compression of the optic nerves and/or chiasm including impaired visual acuity and visual field defects [7]. Our case presented with bitemporal hemianopsia due to compression of the optic chiasm similar to other suprasellar tumors. Our patient did not have any clinical and biochemical evidence of endocrinopathy except for low testosterone before surgery. Pituicytomas have been described as solid sellar and/or suprasellar masses, typically round or oval in shape. The MRI scans generally show isointense on T1-weighted images, and hypointense or slightly hyperintense on T2-weighted images. They have been presented as strong enhancement after administration of contrast agents [278]. Marked homogenous enhancement demonstrates a highly vascular tumor. Most of the reported cases in the literature were primarily interpreted as pituitary macroadenomas or meningiomas as in our case. They can exhibit sellar enlargement and bony remodeling similar to changes of the sellar floor by pituitary macroadenomas. In cerebral angiography of pituicytomas, Gibbs et al. [9] showed that a tumor was visible during the venous phase of selective internal carotid angiography, and even visible during the late venous phase. The pituicytoma demonstrated rapid homogenous enhancement in the early phase of angiography, rather than the gradual enhancement commonly seen with pituitary adenomas [1011]. The rapid enhancement correlates with the increased vascularity of pituicytomas, which is corroborated in our case as well as in the patient with significant vascular blush on the angiogram of the case reported by Gibbs et al. [9] Dynamic contrast-enhanced MRI scans can assist in generating a differential diagnosis from other sellar and suprasellar lesions such as pituitary adenomas and meningiomas [12]. The radiological differential diagnosis includes sellar and suprasellar diseases with enhancement such as pituitary adenomas, meningiomas, pilocytic astrocytomas, granular cell tumors, craniopharyngiomas, gangliogliomas, germ cell tumors, sarcoidosis, and metastatic tumors. Since pituicytomas are extremely rare and their radiological findings are relatively nonspecific, the diagnosis mainly depends on pathological evaluation.
Surgical resection is the most important part of treatment for pituicytomas. In the largest series to date, Brat et al. [3] reported no recurrence of the tumor in patients who underwent total surgical resection. On the other hand, according to the literature [131415], pituicytomas are reported to be much more vascular than other suprasellar tumors. This massive bleeding from hypervascularity during surgery is probably the main reason to explain the high numbers of subtotal resections. In contrast to usual pituitary adenomas, pituicytomas are highly vascular and attached to normal anatomical structures such as the infundibulum or the posterior pituitary lobe, making them difficult to resect completely. Although preoperative embolization of pituicytomas is recommended to increase the chance of a total resection, the tumor in our case was fed from several tiny and uncatheterizable vessels that arise from both internal carotid arteries. Therefore, embolization with particles to decrease hemorrhage during surgery was considered too risky for ischemic stroke from anterograde flow of embolic agents. The choice of surgical approach depends largely on the size and extent of the tumor. Wolfe et al. [6] have advocated a larger craniotomy with extensive cranial base approach in view of the vascularity and firm consistency of the tumor. A staged TSA operation was also recommended for gross total removal of the pituicytoma when diagnosis at the time of the operation is not defined and heavy uncontrolled bleeding occurs [16]. The endoscopic endonasal transsphenoidal transplanum approach could provide gross total removal of the pituicytoma by direct visualization of the surface of tumor and progressive debulking that tends to rapidly devascularize the tumor [17]. The choice of surgical approach depends on the surgeon's preference and ability. However, total removal of the tumor with unusual bleeding may be possible when diagnosis of an uncommon sellar tumor is made preoperatively. The rare event that was encountered in the first TSA of our case was the development of profuse intraoperative bleeding resistant to hemostatic agents. We inevitably decided to stop surgery due to the high bleeding tendency of the tumor and the narrow operative space after obtaining specimen for pathologic examination. We removed the suprasellar tumor by the transcranial approach to decompress the optic nerves and chiasm to prevent permanent visual loss after angiography and recognition of the unusual pathology.
We suggest that pituicytomas should be included as a differential diagnosis of pituitary tumors in patients with homogenously highly-enhanced lesions in the sellar and suprasellar area because of the potential for significant intraoperative bleeding unlike common pituitary adenomas or meningiomas, especially if the surgeon is not aware of this possibility.
Figures and Tables
Fig. 1
Preoperative magnetic resonance imaging. T1-weighted axial image (A) showing slightly hyperintense sellar mass, T2-weighted axial image (B) showing isointense sellar mass, and T1-weighted gadolinium enhanced coronal (C) and sagittal (D) images demonstrating marked enhancement of sellar-suprasellar mass with involvement of the optic nerves and chiasm.
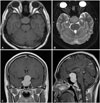
Fig. 2
Angiograms of right (A) and left (B) internal carotid arteries showing significant tumor blush in the arterial phases by multiple small feeders from both internal carotid arteries.
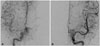
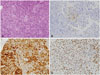
Fig. 3
Histopathological findings of the pituicytoma. A: The tumor cells have moderate to abundant amount of pale eosinophilic cytoplasm and ovoid nuclei with small nucleoli. Necrosis is not identified (hematoxylin and eosin staining, ×400). B: Immunohistochemistry for glial fibrillary acidic protein shows focal positivity (×400). C: The tumor cells are positive for S100 protein (×400). D: The tumor cells show positive staining for thyroid transcription factor 1 (×400).
References
1. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.

2. Liss L, Kahn EA. Pituicytoma, tumor of the sella turcica; a clinicopathological study. J Neurosurg. 1958; 15:481–488.
3. Brat DJ, Scheithauer BW, Staugaitis SM, Holtzman RN, Morgello S, Burger PC. Pituicytoma: a distinctive low-grade glioma of the neurohypophysis. Am J Surg Pathol. 2000; 24:362–368.
4. Lee EB, Tihan T, Scheithauer BW, Zhang PJ, Gonatas NK. Thyroid transcription factor 1 expression in sellar tumors: a histogenetic marker. J Neuropathol Exp Neurol. 2009; 68:482–488.

5. Bara D, Lantos P. Two rare forms of tumour in the hypothalamo-hypophysial system. Infundibular choristoma and glioblastoma infiltrating the pituitary. Acta Morphol Acad Sci Hung. 1968; 16:243–250.
6. Wolfe SQ, Bruce J, Morcos JJ. Pituicytoma: case report. Neurosurgery. 2008; 63:E173–E174. discussion E174.
7. Secci F, Merciadri P, Rossi DC, D'Andrea A, Zona G. Pituicytomas: radiological findings, clinical behavior and surgical management. Acta Neurochir (Wien). 2012; 154:649–657.

8. Brat DJ, Scheithauer BW, Fuller GN, Tihan T. Newly codified glial neoplasms of the 2007 WHO Classification of Tumours of the Central Nervous System: angiocentric glioma, pilomyxoid astrocytoma and pituicytoma. Brain Pathol. 2007; 17:319–324.

9. Gibbs WN, Monuki ES, Linskey ME, Hasso AN. Pituicytoma: diagnostic features on selective carotid angiography and MR imaging. AJNR Am J Neuroradiol. 2006; 27:1639–1642.
10. Katsuta T, Inoue T, Nakagaki H, Takeshita M, Morimoto K, Iwaki T. Distinctions between pituicytoma and ordinary pilocytic astrocytoma. Case report. J Neurosurg. 2003; 98:404–406.
11. Miki Y, Matsuo M, Nishizawa S, et al. Pituitary adenomas and normal pituitary tissue: enhancement patterns on gadopentetate-enhanced MR imaging. Radiology. 1990; 177:35–38.

12. Tian Y, Yue S, Jia G, Zhang Y. Childhood giant pituicytoma: a report and review of the literature. Clin Neurol Neurosurg. 2013; 115:1943–1950.

13. Furtado SV, Ghosal N, Venkatesh PK, Gupta K, Hegde AS. Diagnostic and clinical implications of pituicytoma. J Clin Neurosci. 2010; 17:938–943.

15. Phillips JJ, Misra A, Feuerstein BG, Kunwar S, Tihan T. Pituicytoma:characterization of a unique neoplasm by histology, immunohistochemistry, ultrastructure, and array-based comparative genomic hybridization. Arch Pathol Lab Med. 2010; 134:1063–1069.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download