Abstract
Background
Alongside the extent of removal and patients’ survival in the management of brain tumors, health-related quality of life (HRQOL) has become an important consideration. The purpose of this study is to evaluate the change of HRQOL in brain tumor patients before and after surgery and to assess the associated factors that contribute to the change of HRQOL.
Methods
A total of 258 patients who underwent surgical treatment were enrolled in this study. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30 (EORTC QLQ-C30) and the 20-item EORTC QLQ-Brain Neoplasm (QLQ-BN20) were used to assess HRQOL. Patients were asked to fill out the questionnaires before and 3-6 months after surgery.
Results
Global QOL (p<0.001) and emotional function (p<0.018) were significantly improved after surgery. Physical function (p=0.015) was significantly aggravated. Among the symptoms, headache, pain and nausea and vomiting were significantly decreased (p<0.01, p=0.041, p<0.001, respectively), while dyspnea, communication deficit and weakness of the legs were increased (p=0.005, p=0.040, and p=0.014, respectively). Preoperative neurologic deficit (p=0.019) and tumor diameter (p=0.016) were significantly related to the patients who showed aggravation of global QOL after brain tumor surgery. In the aggravated global QOL group, common complaints and concerns included role function, appetite loss, financial difficulty and future uncertainty.
Conclusion
In brain tumor patients, HRQOL has improved after surgery. Role function, appetite loss, financial difficulty and future uncertainty were important factors for HRQOL in brain tumor patients treated with surgery. Although there is National Health Insurance and Medical Aid program in Korea, financial difficulty and future uncertainty are much more important in influencing QOL than previously thought. The results of this short-term follow up preliminary study suggest that several factors were related to HRQOL, Further research is needed to evaluate the long term change of HRQOL and enhance the global QOL by analyze related factors.
Traditionally, the goals of brain tumor management included patients’ overall and progression-free survival, as well as gross total removal of tumors. Accordingly, physicians up until recently have focused on prolonging the disease-free state of patients rather than improving their health-related quality of life (HRQOL). Among the treatment modalities, surgical tumor removal has been the standard for brain tumor management. However, this treatment option often resulted in various degrees of postoperative complications, leading to the deterioration of physical, emotional, and social functions. This can ultimately result in a reduction of HRQOL for brain tumor patients.
However, there has been a recent shift in the paradigm of healthcare good health, according to this new paradigm, not only means the absence of disease, but also includes physical, social, and mental well-being of an individual [1]. Moreover, in line with this new perspective, the main objective of brain tumor management had been expanded to include not only increasing the overall survival, but also improving HRQOL. Therefore, treatment strategies for brain tumors must focus on enhancing patients’ HRQOL.
Although there exist several studies regarding postoperative HRQOL of brain tumor patients in the literature, there lacks HRQOL assessment of brain tumor patients in our country [2]. This study was planned to evaluate the change of HRQOL in brain tumor patients before and after surgery and to assess the associated factors that influence the change of HRQOL.
A total of 326 patients diagnosed with brain tumor, who underwent surgical treatment at Seoul National University Bundang Hospital (SNUBH), were initially enrolled in this study between February 2012 and November 2015. Patients were asked to fill out a questionnaire assessing the HRQOL and symptoms before the surgery. The same questionnaire was filled out by each participant 3–6 months after the surgery, as the first follow-up assessment. The patients were asked to complete the self-administered questionnaire from either the outpatient clinic or the inpatient wards.
Sixty-eight patients were eliminated from analysis: 60 patients were either lost during the follow-up period or declined to answer the questionnaire, and 8 patients were in a mental state that was not conducive to answering the questionnaire. All participants provided written informed content. The study approved by the Institutional Review Board at SNUBH (B-1202-145-305).
Patient information in the database included demographic data (name, sex, age, preoperative symptom, preoperative neurologic deficit, date of surgery, surgery type, tumor pathology, postoperative complication, and adjuvant therapy). Tumor pathology was classified as glioblastoma, meningioma, pituitary adenoma, vestibular schwannoma, metastasis, and other malignant and benign tumors. Patient information also included tumor diameter (maximal tumor diameter at preoperative MRI) and the extent of tumor resection (gross total removal, subtotal removal, partial removal, and biopsy).
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30 (EORTC QLQ-C30) [3] and the 20-item EORTC QLQ-Brain Neoplasm (QLQ-BN20) [4] were used to assess HRQOL. We evaluated and analyzed the change of QOL, as well as the factors that influenced it, in brain tumor patients after surgery.
Patients completed the EORTC questionnaires before surgery and 3–6 months after surgery. The QLQ-C30 questionnaire was comprised of 5 functional scales (physical, role, emotional, cognitive and social), 3 symptom scales (fatigue, nausea and vomiting, and pain), 6 single-item scales (dyspnea, insomnia, appetite loss, constipation, and financial effect on treatment), and a global QOL. The QLQ-BN20 questionnaire consisted of 11 items, grouped into 4 domains (future uncertainly, visual disorder, communication deficit, and motor dysfunction) and 7 single-items (headache, seizure, drowsiness, hair loss, itching, weakness of both legs, and difficulty controlling bladder function).
QLQ-C30 was scored according to the recommended EO-RTC procedures. All raw scores were converted to be within a range between 0 and 100. For the functioning scales and the global QOL scale, a higher score indicated better functioning; whereas for the symptom scales/items, a higher score indicated a greater degree of symptom or difficulty. The QLQ-BN 20 was scored in a manner analogous to the QLQ-C30; higher scores indicated a greater degree of symptom or difficulty.
Statistical analyses were made by SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). QOL scores were expressed as the mean and standard deviation, and p-values if needed. The numerical comparisons between the data were assessed by t-test and Wilcoxon test in pairwise comparisons. The pairwise comparisons between the two independent groups were assessed by t-test and Mann-Whitney U test. To analyze the related factors for improved QOL, the study population was divided in two groups—aggravated HRQOL and not-aggravated HRQOL—depending on the global QOL change after surgery. Their relationship was assessed by Fisher’s exact test and logistic regression analysis. A p-value of <0.05 was considered significant.
In total, 258 patients were evaluated. Table 1 shows the baseline demographic characteristics. The median age was 49 years (range: 11–76 years); 109 patients were male and 149 patients were female. The extent of resection included gross total tumor (100% macroscopic removal of the tumor mass), subtotal resection (not 100%, but as ≥90%), partial resection (<90%), and open biopsy. It was based on immediate postoperative MRI findings.
The mean baseline scores are shown in Table 2. In baseline QOL, the emotional function suffered the most impairment among the baseline functional scales. Symptoms of fatigue and headache were the most common among the baseline symptom scales.
We analyzed patient’s factors related to decreasing baseline domains. The old age group (>60 years), we observed greater impairment in physical function (p=0.040) and weakness of legs (p=0.049) compared with the young age group. The young age group complained of headache more than the old age group (p=0.022). In females, global QOL (p=0.032) and emotional function (p=0.016) were more impaired than their male counterparts. Females complained of fatigue (p=0.09), nausea and vomiting (p=0.027), pain (p=0.01) and headache (p=0.016) more than males. Moreover, patients with a tumor diameter of greater than 5 cm severely complained of global QOL (p=0.045), visual disorder (p=0.040), and weakness of legs (p=0.018). In patients with preoperative neurologic deficit, global QOL (p<0.031) and role function (p=0.028) were more impaired.
After surgery, global QOL was remarkably improved by 8.3 points (p<0.001) (Table 2, Fig. 1). Emotional function (p<0.018) was also improved by 4.3 points. However, physical function was aggravated over time, with a difference in the mean score of 3.3 points between the baseline and 3–6 months follow-up (p=0.015). Other functional domains did not show significant p values.
Effective domains of improved symptoms were headache, nausea and vomiting, and pain. Their score changes were 10.1, 5.1, and 3.9 points, respectively (p<0.01, p=0.041, p<0.001). However, dyspnea, communication deficit, and weakness of legs were worsened (p=0.005, p=0.040, and p=0.014, respectively).
In case of meningioma and metastasis, global QOL was improved remarkably (Fig. 2), with score changes of 12 and 22 points respectively (p=0.025, SD=35.5, and p<0.001, SD=27.4, respectively). But in the case of vestibular schwannoma, there was only an improvement of 2 points (p=0.20, SD=30.6). No significant global QOL changes were seen with respect to preoperative neurologic deficit or operation type. Global QOL was improved more in case of gross total resection compared with subtotal, partial, or biopsy. In gross total resection, global QOL was improved by 10 points compared with 6 points in partial resection or biopsy.
We analyzed the related factors for aggravated global QOL by chi-square test and logistic regression analysis, as shown in Table 3. In evaluating the related factors for global QOL, meaningful change in the score was defined by a score change of more than 10 points [5]. In multivariate study, age, male, preoperative neurologic deficit, adjuvant therapy, postoperative chemotherapy, postoperative complication and tumor diameter were analyzed. Patients without preoperative neurologic deficit showed more aggravated global QOL [p=0.019, odd ratio (OR) 2.05]. Tumor diameter of less than 5 cm was a factor related to aggravated global QOL (p=0.016, OR 2.80). Age, sex, postoperative complication, adjuvant therapy, and surgery type (craniotomy, trans-sphenoidal approach, or biopsy) were independent for improved global QOL.
We analyzed other scales in the aggravated global QOL group. In this group, physical function (p<0.01), cognitive function (p=0.016), social function (p=0.007), and role function (p<0.001) were aggravated. In these, the role function was the most aggravated functional domain. Appetite loss (p=0.012), financial difficulty (p=0.029), future uncertainty (p<0.001), and motor dysfunction (p<0.001) were domains with the most complaints. Constipation was the only improved symptom.
In this study, we evaluated the baseline HRQOL and the 1st follow up HRQOL after surgery. This study was designed to understand the changes in various domains of HRQOL and to analyze the related factors for HRQOL. In doing so, we hope that it could help physicians to choose the best treatment option for the improvement of HRQOL.
In some studies, the baseline HRQOL serves as an independent prognostic factor for survival or locoregional control. In other studies, it was found that HRQOL scores were related with survival [678].
In this study, female patients showed a greater impairment of global QOL than male patients. In previous studies, it was reported that female brain tumor patients tended to have lower HRQOL than male patients [9]. The exact etiology of this is unclear; however, it would be plausible to assert that female attributes—greater anxiety, mood changes, and overall psychological profiles—may have an association with lower HRQOL [10].
No significant global QOL changes were seen with respect to preoperative neurologic deficit. The exact etiology of this is unclear; but, other studies showed same result [1112].
In case of vestibular schwannoma, no significant global QOL changes were seen after surgery. This may have been the case due to postoperative neurologic changes in vestibular schwannoma [13].
Headache and pain were improved after surgery. This may be due to decreased mass effect [14]. However, itchy skin, hair loss, and weakness of legs were worsened. This might be the effects of hair shaving and postoperative chemotherapy [15].
It is important to realize that HRQOL is not only important as a standalone measurement, but it also serve as a predictor of overall survival. The baseline QOL, including cognitive function, was highly predictive of overall survival [1617]. Moreover, other studies reported that a decreased QOL is associated with shorter survival in long-term follow-up [7]. Hence, sincere efforts to improve HRQOL are crucial in the management of brain tumor patients.
In aggravated global QOL group, role function, financial difficulty and future uncertainty were domains with the most complaints. Patients undergoing treatment for brain tumor have reported a variety of work-related problems including job loss, undesired change in their job responsibilities, and diminished work capacity, which worsens their financial burden. Although there is National Health Insurance and Medical Aid program in Korea, financial difficulty and future uncertainty are much more important in influencing QOL than previously thought. Also, Cognitive changes associated with disease or treatment related neurologic dysfunctions are amplified by comorbid psychosocial problems such as job loss and financial difficulties.
This study has some limitations to consider. First, a short follow-up period, of only 3–6 months. Second, 326 patients were enrolled at the baseline, but 60 patients were eliminated at the 3–6 months follow-up due to either loss of follow-up, patient’s refusal to participate, or aggravated neurologic condition. Thus, it could reflect a selection bias. Third, although we assessed cognitive function with the EORTC QLQ-C30, we did not assess cognitive function with cognitive test batteries, and it is known that the self-report of functioning and formal neurocognitive testing may be poorly correlated [18].
In conclusion, HRQOL has improved after surgery in brain tumor patients treated with surgery. Emotional function, headache, and pain were improved most. Preoperative neurologic deficit and tumor diameter were significantly related to the patients who showed aggravation of global QOL after brain tumor surgery.
Role function, financial difficulty and future uncertainty were important factors for HRQOL in brain tumor patients treated with surgery. Although there is National Health Insurance and Medical Aid program in Korea, financial difficulty and future uncertainty are much more important in influencing QOL than previously thought.
The results of this short-term follow up preliminary study suggest that several factors were related to HRQOL, Further research is needed to evaluate the long term change of HRQOL and enhance the global QOL by analyze related factors.
Figures and Tables
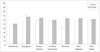 | Fig. 2Global QOL change after surgery according to pathologic diagnosis. *p<0.05. QOL, quality of life. |
Table 1
The baseline clinical characteristics (n=258)

Table 2
Baseline and postoperative HRQOL

Table 3
Related factor for aggravated Global QOL (By chi-square test & logistic regression analysis)

References
1. Larson JS. The World Health Organization’s definition of health: social versus spiritual health. Soc Indic Res. 1996; 38:181–192.

2. Park HJ, Yang HK, Shin DW, et al. Cross-cultural adaptation of the korean version of the minneapolis-manchester quality of life instrument-adolescent form. J Korean Med Sci. 2013; 28:1788–1795.

3. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85:365–376.

4. Taphoorn MJ, Claassens L, Aaronson NK, et al. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010; 46:1033–1040.

5. Minniti G, Scaringi C, Baldoni A, et al. Health-related quality of life in elderly patients with newly diagnosed glioblastoma treated with short-course radiation therapy plus concomitant and adjuvant temozolomide. Int J Radiat Oncol Biol Phys. 2013; 86:285–291.

6. Bosma I, Reijneveld JC, Douw L, et al. Health-related quality of life of long-term high-grade glioma survivors. Neuro Oncol. 2009; 11:51–58.

7. Mainio A, Tuunanen S, Hakko H, et al. Decreased quality of life and depression as predictors for shorter survival among patients with low-grade gliomas: a follow-up from 1990 to 2003. Eur Arch Psychiatry Clin Neurosci. 2006; 256:516–521.

8. Khan F, Amatya B. Factors associated with long-term functional outcomes, psychological sequelae and quality of life in persons after primary brain tumour. J Neurooncol. 2013; 111:355–366.

9. Mainio A, Hakko H, Niemelä A, Koivukangas J, Räsänen P. Gender difference in relation to depression and quality of life among patients with a primary brain tumor. Eur Psychiatry. 2006; 21:194–199.

10. Cheng JX, Zhang X, Liu BL. Health-related quality of life in patients with high-grade glioma. Neuro Oncol. 2009; 11:41–50.

11. Boele FW, Heimans JJ, Aaronson NK, et al. Health-related quality of life of significant others of patients with malignant CNS versus non-CNS tumors: a comparative study. J Neurooncol. 2013; 115:87–94.

12. Tsay SL, Chang JY, Yates P, Lin KC, Liang SY. Factors influencing quality of life in patients with benign primary brain tumors: prior to and following surgery. Support Care Cancer. 2012; 20:57–64.

13. Sanna M, Taibah A, Russo A, Falcioni M, Agarwal M. Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol. 2004; 25:379–386.

14. Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003; 361:323–331.

15. Bae SH, Park MJ, Lee MM, et al. Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. J Korean Med Sci. 2014; 29:980–984.

16. Cella D. Beyond traditional outcomes: improving quality of life in patients with renal cell carcinoma. Oncologist. 2011; 16:Suppl 2. 23–31.

17. Corn BW, Moughan J, Knisely JP, et al. Prospective evaluation of quality of life and neurocognitive effects in patients with multiple brain metastases receiving whole-brain radiotherapy with or without thalidomide on Radiation Therapy Oncology Group (RTOG) trial 0118. Int J Radiat Oncol Biol Phys. 2008; 71:71–78.

18. Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013; 31:65–72.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download