Abstract
Primary meningeal hemangiopericytoma (HPC) is a rare, aggressive dura based tumor that remarkably mimics a meningioma clinically and radiologically. Its occurrence within the cerebellopontine angle (CPA) is exceptional, and establishing the exact diagnosis is of the utmost importance since total resection remains the cornerstone of treatment. A 42-year-old man presented with a three-month history of progressively worsening vertigo and difficulty in walking. On admission, his neurological examination revealed a right peripheral facial palsy, right abducens palsy and left hemiparesis, suggesting the diagnosis of Millard-Gubler syndrome. Computed tomography and magnetic resonance imaging demonstrated a homogeneously enhancing dura based lesion of the right CPA causing major brain stem compression. There was no widening of the ipsilateral internal auditory canal. A standard retrosigmoid craniotomy was performed to access the right CPA. Exposure of the lesion revealed a well-encapsulated, gray, fibrous lesion, which appeared to originate from the tentorium. Gross total resection was achieved and confirmed radiologically. The microscopic features and the immunohistochemical profile confirmed the diagnosis of a HPC, and adjuvant radiation therapy was administered. Ten years later, the patient presented with a severe neurological deficit due to a local recurrence, but at that time refused any second intervention. He died three months later. HPC can locate within the CPA and present as a Millard-Gubler syndrome. The diagnosis should be kept in mind in case of a CPA dura based tumor. Radical surgery plus radiation therapy can maximize the recurrence-free survival and close follow-up remains mandatory to spot recurrences early.
Hemangiopericytomas (HPCs) are rare, and are aggressive neoplasms that most often involve the musculoskeletal system and skin [1]. Their occurrence within the cerebellopontine angle (CPA) is exceptional [23456789], but given the considerable overlap of clinical, radiological and pathological features between meningiomas, solitary fibrous tumors (SFTs) and HPCs, and the very aggressive behavior of HPC compared to its counterparts; the differentiation becomes of the utmost importance.
We describe herein an unusual case of a CPA HPC presenting as a right Millard-Gubler syndrome that recurred ten years after radical surgery plus radiation therapy.
The patient was a 42-year-old male who presented in June 2004 with complaints of vertigo and dysequilibrium while walking that evolved for four months. He also complained of a slowly aggravating right facial weakness for 3 months associated with ipsilateral facial numbness.
On admission, his neurological examination revealed a right peripheral facial palsy, right abducens palsy, and left hemiparesis, suggesting the presence of the Millard-Gubler syndrome.
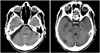
Head CT scan and an MRI of the brain demonstrated a homogeneously enhancing 2.8×3.5 cm sized right CPA lesion causing major brain stem compression without extension into the ipsilateral internal acoustic meatus (Fig. 1).
As the tumor seemed to be dura based, the preoperative diagnosis of a meningioma of the CPA was considered, even though neither dural tail sign nor hyperostosis was observed.
A suboccipital retrosigmoid approach was attempted. During surgery, the tumor was prone to bleeding, and which arose between the Vth and the VII/VIIIth nerve complex with a clear dural basement and no connection with the cranial nerves. There was no tumor within the internal auditory meatus, and although it was fleshy and non suckable, it yielded to cavitron ultrasonic surgical aspirator. Somatosensory evoked potentials, brainstem auditory evoked responses, and intraoperative facial nerve monitoring were used to ensure the safe separation of tumor from the brainstem and associated cranial nerves. Simpson grade II excision was achieved and confirmed on post-operative CT scan (Fig. 2).
The patient recovered quickly with no facial nerve paresis, and was discharged home on the 4th post-operative day.
The histopathology sections revealed a richly vascular tumor with multiple foci of cells with distinct and indistinct cell borders interspersed with small arborizing capillaries. Large irregular blood vessels (staghorn like) were also noticed. The nuclei were round to oval with minimal atypia and few mitotic figures. No necrosis could be observed. Mitotic activity was low but the Ki-67 labeling index was 7%. The cells expressed positivity for vimentin staining and were negative for epithelial membrane antigen. CD34 expression was strongly positive. The morphological and immunohistochemical features strongly favored the diagnosis of a grade II HPC (Fig. 3, Fig. 4).
Metastatic workup including CT of the chest/abdomen/pelvis did not reveal any evidence of extracranial HPC. Adjunctive radiation therapy was administered and a close clinical and radiological follow-up established. He was lost to follow up since March 2008.
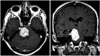
Six years later, the patient was re-admitted to our department with headache and vomiting. At the time of admission, his physical examination revealed a left hemiparesis, and control MRI of the brain showed local recurrence (Fig. 5). The patient refused any secondary intervention and no adjunctive treatment was administered. He died three months later.
HPCs are rare, and are aggressive neoplasms that arise from the pericytes of Zimmerman, which are contractile spindle cells surrounding capillaries and post capillary venules [4]; and most often involve the musculoskeletal system and skin [1].
Intracranial HPCs represent only 0.4% of all intracranial tumors [10] and approximately 2% to 4% of all meningeal tumors [5].
Unlike meningiomas, HPCs tend to occur more often in males, with a male-female ratio approaching 2:1 and a mean age of presentation in the fifth decade [11]. The extreme rarity of HPCs at CPA, however, precludes any demographic markers such as age or sex predilection (Table 1).
Since HPCs are difficult to differentiate radiographically from other skull base tumors such as meningiomas, SFTs, and schwannomas; some authors such as Salunke et al. [8] insist on the importance of preoperative planning in suspicious cases of CPA dura based masses and lesions showing disproportionate perilesional edema, narrow base of attachment, or multilobulated cross-leaf growth [12]. Other authors have proposed subtle imaging characteristics that may help to distinguish HPCs from meningiomas, such as the absence of calcification or bony hyperostosis, or the comparison of the apparent diffusion coefficient values in peritumoral edema [813]. Recently, positron emission tomography has emerged as a potentially useful diagnostic tool for differentiating HPCs from meningiomas, but its high cost and availability do not permit routine use in the initial radiological investigation of CPA dura-based lesions. Consequently, the most reliable tool in HPC diagnosis remains as accurate immunohistochemical workup, and although psammoma body and nuclear pseudo inclusions can be found in meningiomas, epithelial membrane antigen negativity and CD34/CD99 positivity assists in the differentiation [5].
Another standpoint is excluding HPC from fibrous variants of SFT (conventional SFT), which have been commonly described to occur at the CPA [14]. Lately, these two entities (SFT and HPC) were believed to represent two parts of the spectrum with fibrous SFT at one end and cellular SFT at the other end. The current World Health Organization classification also acknowledges the overlap, and states these two entities as part of the spectrum [15]. The cellular variant of SFT has lately been proposed to be synonymous with HPC due to considerable morphological and immunohistochemical overlapping [7]. Immunoreactivity for CD34 which is strongly expressed in SFT alone cannot be taken as its diagnostic marker, as 40% of HPC also show reactivity with CD34 [7], and the subunit A of factor XIII once documented as a diagnostic marker for HPC is focally positive in SFT [27]. Therefore, careful histopathological examination is helpful in distinguishing the fibrous variants of SFT from HPC. SFT shows wavy fascicles of elongated undulating cells associated with collagenous bands, whereas HPC shows closely packed, randomly oriented cells with little fibrosis with staghorn sinusoidal vessels and fine reticulin pattern. The neoplastic cells in HPC are also closely packed with little intervening fibrosis.
In the present case, the morphological features were distinctive enough to place the lesion in the category of an HPC. Fairly uniform cellularity of the tumor and the low mitotic activity was more supportive of this lesion being labeled as grade II HPC instead of a cellular SFT.
Unfortunately, the similarities between meningiomas and HPCs end at their radiographic and gross characteristics, as the typical indolent behavior exhibited by most low-grade meningiomas is in stark contrast with the aggressive behavior observed in most cases of HPC. In fact, with a mean survival of 84 months from the time of initial diagnosis [5], a local recurrence rate as high as 91% and a 15-year risk of distant metastasis approaching 70%; intracranial HPCs harbor one of the most aggressive biological/clinical behaviors [16].
Their management relies consequently on a close cooperation between clinicians, surgeons, and pathologists from establishing diagnosis to organizing the therapeutic strategy.
In the current state of knowledge, radical surgical resection, whenever feasible, followed by radiation therapy can be considered as the optimal management policy. Radiation therapy has in fact extended the mean time of local recurrence from 34 to 75 months, and the survival from 62 to 92 months [1].
Depending on the tumor size, some authors advocate the preoperative use of stereotactic radiotherapy as it is associated with the best disease free survival [5], while some others such as Kumar et al. [1], support its role in recurrent disease or re-irradiation. Cho et al. [6] in 2011 reported a case of recurrent CPA HPC 5 years after stereotactic radiosurgery, and supported the use of stereotactic radiosurgery alone for small tumors with the understanding that the tumor may eventually recur. The ten year survival observed here for the first time supports our preferential aggressive surgical approach combined with radiation therapy. Consequently, although we cannot deny the benefits of the use of stereotactic radiosurgery, we do not support its role as the sole therapeutic option in the initial management of these rare and aggressive tumors.
Chamberlain and Glantz [17] in 2008, reported that a chemotherapy protocol combining: cyclophosphamide+adriamycin+vincristine, followed by α-interferon, followed by ifosamide-carboplatin-etoposide, may be helpful for recurrent intractable cases, but such encouraging results was tempered by other studies that observed no real place for chemotherapy in the available therapeutic armamentarium of intracranial HPCs.
In the present case, no chemotherapy was administered either in the initial management or at recurrence.
In conclusion, given the clearly aggressive nature of intracranial HPCs, it becomes imperative to include them in the differential diagnosis of CPA dura based tumors.
A high index of suspicion on radiology imaging is essential to plan for total excision, and an accurate histopathological diagnostic precision is of the utmost importance.
As postoperative recurrence seems unavoidable, long-term follow-up with serial imaging should be considered in all cases.
Figures and Tables
Fig. 1
Post-Gadolinium magnetic resonance imaging of the brain in axial (A), coronal (B), and sagittal (C) views showing a lobulated right cerebellopontine angle tumor with a tentorial attachment base.
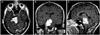
Fig. 3
Photomicrographs of the tumor specimens showing. A: Diffuse sheets of relatively uniform population of cells interspersed by staghorn vascular channels (H&E, original magnification, ×10). B: Round to oval cells with finely dispersed nuclear chromatin and moderate cytoplasm and no signs of anaplasia (H&E, original magnification, ×20). H&E, hematoxylin and eosin.

Fig. 4
Immunohistochemical staining showing diffuse positivity with CD34 (original magnification, ×100).
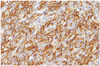
Table 1
Literature review of cerebellopontine angle hemangiopericytomas

| Author (year) | Age | Sex | Clinical presentation | Management | Post-operative course | Outcome |
|---|---|---|---|---|---|---|
| Molnar and Nemes (1995) [2] | 64 | F | Cerebellar involvement | Surgery+RT | Local recurrence | 4 years with metastasis |
| Mallucci et al. (1999) [3] (two cases) | n.m | n.m | Cerebellar involvement | Surgery±RT | No recurrence | n.m |
| Alén et al. (2001) [4] | 12 | F | Hearing loss, facial palsy | Surgery+RT | Multiple recurrences | Death after 76 months |
| Tashjian et al. (2009) [5] | 37 | M | Hearing loss, trijeminal involvement | Surgery+RT | No recurrence | n.m |
| Cho et al. (2011) [6] | 39 | M | Hearing loss, tinnitus | SRS | Recurrence at 5 years (total surgical resection) | Doing well at two years |
| Zeng et al. (2012) [7] | 22 | M | Incidental discovery | Surgery+RT | No recurrence | Doing well at one year |
| Salunke et al. (2013) [8] | 63 | M | Hearing loss, facial palsy | Surgery+RT | No recurrence | Doing well at six months |
| Teoh et al. (2014) [9] | 24 | F | Hearing loss, facial palsy, trigeminal involvement, tinnitus and headache | Surgery+RT | Stable remnant | Doing well at one year |
| Present case | 42 | M | Millard-Gubler syndrome | Surgery+RT | Recurrence at 10 years | Refused re-intervention |
References
1. Kumar N, Kumar R, Kapoor R, et al. Intracranial meningeal hemangiopericytoma: 10 years experience of a tertiary care Institute. Acta Neurochir (Wien). 2012; 154:1647–1651.

2. Molnar P, Nemes Z. Hemangiopericytoma of the cerebello-pontine angle. Diagnostic pitfalls and the diagnostic value of the subunit A of factor XIII as a tumor marker. Clin Neuropathol. 1995; 14:19–24.
3. Mallucci CL, Ward V, Carney AS, O'Donoghue GM, Robertson I. Clinical features and outcomes in patients with non-acoustic cerebellopontine angle tumours. J Neurol Neurosurg Psychiatry. 1999; 66:768–771.

4. Alén JF, Lobato RD, Gómez PA, et al. Intracranial hemangiopericytoma: study of 12 cases. Acta Neurochir (Wien). 2001; 143:575–586.

5. Tashjian VS, Khanlou N, Vinters HV, Canalis RF, Becker DP. Hemangiopericytoma of the cerebellopontine angle: a case report and review of the literature. Surg Neurol. 2009; 72:290–295.

6. Cho JM, Kim SH, Kim SH, Lee KS, Chang JH. Recurred cerebellopontine angle haemangiopericytoma 5 years after stereotactic radiosurgery. Clin Neurol Neurosurg. 2011; 113:931–933.

7. Zeng J, Ogera P, Benardete EA, Nicastri AD, Rao C. Cellular solitary fibrous tumor (hemangiopericytoma) with anaplasia at cerebellopontine angle--a case report. Pathol Res Pract. 2012; 208:493–496.

8. Salunke P, Futane S, Gupta K, Vasishta RK. Cerebello-pontine angle hemangiopericytoma: an orphan differential diagnosis. Clin Neurol Neurosurg. 2013; 115:1184–1186.

9. Teoh JW, Goh BS, Shahizon Azura MM, Siti Aishah MA, Nor Hafliza MS. An unexpected lesion in cerebellopontine angle: hemangiopericytoma. Med J Malaysia. 2014; 69:146–147.
10. Melone AG, D'Elia A, Santoro F, et al. Intracranial hemangiopericytoma--our experience in 30 years: a series of 43 cases and review of the literature. World Neurosurg. 2014; 81:556–562.

11. Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer. 2004; 100:1491–1497.

12. Zhou JL, Liu JL, Zhang J, Zhang M. Thirty-nine cases of intracranial hemangiopericytoma and anaplastic hemangiopericytoma: a retrospective review of MRI features and pathological findings. Eur J Radiol. 2012; 81:3504–3510.

13. Liu L, Yin B, Geng DY, Li Y, Zhang BY, Peng WJ. Comparison of ADC values of intracranial hemangiopericytomas and angiomatous and anaplastic meningiomas. J Neuroradiol. 2014; 41:188–194.

14. Chen H, Zeng XW, Wu JS, et al. Solitary fibrous tumor of the central nervous system: a clinicopathologic study of 24 cases. Acta Neurochir (Wien). 2012; 154:237–248. discussion 248.

15. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download