Abstract
Background
Direct surgery to resect tumors in the motor cortex could improve neurological symptoms or cause novel motor weakness. The present study describes the neurological outcomes of patients after the surgical resection of non-glial tumors in the primary motor cortex.
Methods
The present study included 25 patients who had pathologically confirmed non-glial tumors in the motor cortex for which they underwent surgery. Tumor location was verified using anatomical landmarks on preoperative magnetic resonance imaging scans. All surgeries involved a craniotomy and tumor resection, especially use of the sulcal dissecting approach for intra-axial tumors.
Results
Of the 25 patients, 10 exhibited metastasis, 13 had a meningioma, and 2 had a cavernous malformation. Motor weakness and seizures were the most common symptoms, while 3 patients experienced only a headache. The tumor size was less than 20 mm in 4 patients, 20–40 mm in 14, and greater than 40 mm in seven. Of the 25 patients, 13 exhibited motor weakness prior to the operation, but most of these symptoms (76.9%) improved following surgery. On the other hand, eight patients experienced seizures prior to the surgery, and in three of these patients (37.5%), the seizures were not controlled after the surgery. In terms of surgical complications, a postoperative hematoma developed in one of the meningioma patients, and the patient's hemiparesis was aggravated.
The use of surgical treatments for the resection of tumors in the primary motor cortex (PMC) has been challenged because it may result in permanent motor weakness. Thus, various tools such as neuronavigation, cortical mapping, and evoked electrophysiological monitoring have been applied to maximize the safety of marginal resections [123]. Additionally, awake surgery has been performed in such cases to preserve motor function, but this technique is typically associated with glial tumors that have naturally infiltrated the surrounding tissues [45]. In other cases, only a biopsy was advocated.
Non-glial tumors, such as metastatic brain tumors and cavernous malformations, are believed to be delimitated from the surrounding tissue, and the associated neurological symptoms often markedly improve following surgical resection. In fact, some reports have recommended aggressive resection in the case of neurological deficits [67]. The removal of extra-axial tumors is associated with a greater opportunity for neurological improvements without injury to the surrounding brain tissue [8]. Thus, it is likely that the resection of non-glial tumors can be performed without causing damage to the brain. However, few studies focused on the treatment of metastatic tumors or meningiomas located directly in the PMC have reported the results of surgical resection [19].
Lesions to the motor cortex can result in a variety of neurological symptoms including hemiparesis, gait disturbance, impairment of fine hand function, dysarthria, and seizures. However, these symptoms may be improved after tumor compression in the motor area is relieved. Thus, the present study describes the neurological outcomes of patients after surgical resection of non-glial tumors in the PMC.
With the approval of our Institutional Review Board (IRB; SHCBC_IRB_2016-03-005), the present study retrospectively analyzed a series of 25 consecutive patients suffering from non-glial tumors located in the motor cortex who underwent microneurosurgical resection by a single surgeon. All of the non-glial tumors were defined as intra-axial or extra-axial tumorous lesions, except for glial tumors that originated in the neuroepithelium, and included 13 meningiomas, 10 metastatic brain tumors, and 2 cavernous malformations. The characteristics of the 25 patients were assessed, and the changes between their preoperative symptoms at presentation and postoperative symptoms were assessed. Each tumor was pathologically confirmed, and the tumor size was determined using a contrast-enhanced axial magnetic resonance imaging (MRI) scan obtained prior to the operation. The longest dimension of the mass was measured, and the follow-up periods for all patients were at least 3 months.
Using preoperative brain MRI scans, the motor cortex of each patient was defined using anatomical landmarks that have been shown to have strong correlations with the motor cortex in previous studies [10]. The motor cortex and precentral gyrus were consistently located on a broad-based, knob-like area that had an inverted omega or epsilon shape and was oriented in a posterolateral direction, protruding into the central sulcus. It is also possible to locate the precentral gyrus by identifying the typical courses of the superior frontal sulcus and the precentral sulcus [11] or by following the courses of the anterior horizontal and ascending branches of the Sylvian fissure and the precentral sulcus [12].
All surgeries were conducted under general anesthesia by performing a generous craniotomy beyond the tumor margins using a surgical navigation system (Navigation System II®; Stryker, Kalamazoo, MI, USA). Most of the surgeries for the intra-axial tumors were performed in conjunction with intraoperative monitoring (IOM; ISIS system®, Inomed®, Emmendingen, Baden-Württemberg, Germany) of somatosensory evoked potentials (SEPs) and motor evoked potentials (MEPs) in all extremities. A microscope, microtechniques, and a Cavitron ultrasonic surgical aspirator were used in all surgeries, and the dura was always closed watertight.
In the case of intra-axial tumors, the surgical approach included dissection through either the central sulcus or precentral sulcus rather than directly through an incision in the precentral gyrus. The sulcus to be dissected was chosen based on the shortest distance to the tumor margin (Fig. 1A, B), and the tumor was removed via internal decompression before fine dissection. If there was a severe adhesion between the tumor and normal brain tissue or the cleavage plane disappeared, a minimal tumoral layer was left in place to avoid neurological complications, such as new motor weakness. In the case of extra-axial tumors, the tumor was finely dissected from the circumferential normal parenchyma while preserving the pia mater as much as possible (Fig. 1C).
The present study included 25 patients (14 men and 11 women) with a mean age of 57.8 years (range, 35–82 years) who underwent microsurgical resection for non-glial tumors situated in the PMC. The tumor pathology types included meningiomas, metastatic brain tumors, and cavernous malformations. The pathological diagnoses of the meningiomas consisted of meningothelial (n=7), transitional (n=2), atypical (n=2), fibrous (n=1), and angiomatous (n=1). The metastatic brain tumors typically originated from lung cancer (n=6), and the primary lesions of the other tumors included adenocarcinoma of the colon, squamous cell carcinoma of the esophagus, multiple myelomas, and unknown origins. Tumor size was measured using the contrast-enhanced axial images obtained from preoperative brain MRI scans. The largest tumor was 63.1 mm, and the smallest tumor was 10.6 mm; tumor diameters were less than 20 mm in 4 patients, 20–40 mm in 14 patients, and larger than 40 mm in seven (Table 1).
The preoperative neurological deficits of the 25 patients included motor weakness (n=13, 52.0%), seizures (n=8, 32.0%), dysarthria (n=2, 8.0%), numbness (n=1, 4.0%), and headache (n=3, 12.0%) (Table 2). Three patients exhibited monoplegia in only one upper extremity, with the other extremities intact; no movement or nearly no movement (motor grade: 0–1) was observed in the upper or lower extremities of three patients and one patient, respectively.
In terms of the preoperative neurological symptoms, dysarthria and headache were improved or cured in all patients, and motor weakness symptoms exhibited improvements in 10 of 13 cases. Of the remaining three motor weakness cases, the symptoms remained in two patients and were aggravated in one patient. In three of the eight seizure cases, the seizure symptoms remained after the surgery, and in the six cases with subtotal removal of the tumor, three patients had experienced seizures prior to the surgery; in these three patients, the seizures continued to occur after the surgery. On the other hand, of the five patients who experienced preoperative seizures and underwent a total resection of the tumor, four no longer experienced seizures, while one continued to have seizures (Table 3).
In all cases, attempts were made to remove as much of the tumor as possible, but three of the meningiomas and three of the metastatic brain tumors were removed subtotally; the other 19 tumors were completely resected. In the cases of metastatic brain tumors, all patients underwent radiotherapy as an adjuvant therapy, and radiosurgery was performed after the tumor removal operation in two cases of meningiomas that were only partially removed. Of the reported cases, only two did not show favorable outcomes.
A 58-year-old male presented at our emergency department with sudden weakness of the left leg. An extra-axial mass was found in the right middle parasagittal area, and a 27×33-mm tumor was located in the PMC, with extensive edema around the lesion (Fig. 2A, B). A direct surgical resection was planned using navigation-guided craniotomy, but the tumor was highly vascularized and severely adhesive with the pia mater, so no clear dissection plane between the tumor and the brain could be found. However, attempts were made to remove the tumor radically, and the pathologic diagnosis was microcystic and angiomatous meningioma. A MEP was observed on the exposed motor cortex, complete hemostasis was achieved on the surgical field, and a diagnosis of an angiomatous meningioma was confirmed by the pathologist. Unfortunately, a postoperative intracerebral hematoma developed (Fig. 2C), and the patient's motor weakness was aggravated as plegia of the left ankle and a subtle weakness of the left arm. Surgical aspiration was not attempted due to concerns of further injury to the brain. The motor weakness did not improve, even following active physical therapy, and a brain abscess developed at the site of the hematoma 2 months after surgery (Fig. 2D). The abscess was drained and intravenous antibiotics were administrated for 6 weeks, but the patient's left ankle weakness has remained for 2 years after the tumor surgery.
A 39-year-old male presented at our emergency department with a complex partial seizure, and his radiological images revealed a parasagittal meningioma around the motor cortex (Fig. 3A, B). The tumor partially occupied the parasagittal venous sinus, and a near-total surgical resection was planned, followed by stereotactic radiosurgery the next day if necessary. However, due to severe adhesion, it was necessary to leave the tumor attached to the brain tissue (Fig. 3C), and the pathologic diagnosis was meninothilial meningioma. Subsequently, Cyberknife radiosurgery was performed with 210 cGy as the marginal dose. Following the radiosurgery, the tumor size has not reduced, but a slight growth into the cortex was observed (Fig. 3D). The patient's seizures have not remitted for 3 years, and he is currently being treated with levetiracetam (1,500 mg) and sodium valproate (900 mg).
Although not encapsulated, metastatic brain tumors are usually well circumscribed and can often be totally demarcated from the surrounding normal brain parenchyma. Even if a metastatic brain tumor infiltrates the neighboring brain tissue, it is less infiltrative than a glial cell tumor [131415], and its solidity and color differ from normal brain tissue. It is possible to use these characteristics to differentiate metastatic brain tumors from other neural tissues [16], and as a result, the total macroscopic removal of metastatic brain tumors is often easier and less damaging to the circumscribed brain tissue than is the removal of a glial cell tumor [131415]. Cavernous malformations are located in the thin-walled vascular sinusoidal space rather than in normal brain tissue or ventricle. The sinusoids are surrounded by hemosiderin deposits and lined by a thin endothelium that is lacking elastin, intervening parenchyma, and smooth muscle [16171819202122]. Meningiomas are representative of extra-axial intracranial tumors characterized by an arachnoid plane between the tumor and the brain [2324]. If extra-axial tumors are resected without violating the arachnoid plane, the brain is not necessarily injured. Thus, non-glial tumors (like the tumors described above) may be removed with minimal brain injury and without inducing any neurological symptoms. In the present study, the patients' motor symptoms typically improved after surgery, except for one case who suffered a postoperative hematoma.
Tumor masses compress neighboring neural tissues and often occur at the expense of intercellular water, myelin, and glia, or even neuronal cell parameters such as blood oxygen level-dependent functions [8]. These changes may manifest secondary to changes in hemodynamics rather than due to the loss or regeneration of neurons in the PMC [825]. Therefore, the neurological symptoms caused by non-glial tumors can be improved by the removal of the tumor as long as there is no damage to neighboring normal brain tissue during the tumor resection [8]. Most of the patients with motor weakness in the present study experienced an improvement in symptoms following tumor resection.
It is recommended that IOM be performed during tumor resection in the PMC to reduce the likelihood of new neurological symptoms. The central sulcus can be identified using the phase reversal of SEPs [26] or by the application of direct electrical stimulation (DES) to the motor cortex [4]. However, if the use of DES is not possible, the phase reversal of SEPs is the only way to locate the central sulcus [2728]. It is generally believed that metastatic brain tumors are non-infiltrative, and IOM during the resection of a tumor in the PMC is still not widely prescribed. However, recent studies have shown that metastatic brain tumors can infiltrate the surrounding brain tissue; thus, it may be helpful to reduce surgery-related motor deficits by employing the intraoperative surveillance of motor systems. Additionally, IOM, particularly the monitoring of MEPs, reduces the rate of surgery-related neurological deficits, while potentially increasing the extent of resection [29].
In terms of the surgical resection of meningiomas, the arachnoid plane between the tumor and brain allows for easy dissection of the meningioma. However, some cases of meningioma are associated with brain invasion characterized by an irregular border between the brain and the tumor that does not have intervening leptomeninges [23]. Furthermore, a lost cleavage plane could be found during tumor resection. To entirely preserve the function of the PMC in these situations, the tumors should be removed only in an intracapsular manner, and the tumoral layers attached to the cortex should remain as thin as possible because thinner tumoral layers would lower the MEP threshold. An intracapsular tumor resection should be stopped if the difference in MEP threshold between direct cortical stimulation and stimulation through the tumoral layer is 2 mA or lower [1]. In one of the cases described in the present study, new symptoms of motor weakness were induced by surgery for a meningioma because a total resection was attempted even though there was pial adhesion (Fig. 2). However, as in the illustrated case, the seizures would not remit if the meningioma is not totally resected (Fig. 3). Furthermore, when using IOM during the resection of a tumor in the PMC, it is important to remember that preoperative motor weakness due to a tumor in this area may reduce the accuracy of the IOM.
The performance of an awake surgery is one method that can be used to monitor the development of new motor symptoms during surgery in the motor cortex, and awake mapping has frequently been used in recent brain tumor surgeries. In terms of a map of neural connections, the brain of each patient is affected differently by each tumor and is reorganized individually; therefore, the cortical areas associated with motor activity differ from patient to patient. During stimulation of areas around the tumor, if the patient stops performing tasks such as hand movements or counting to 10, then that area should be left alone. However, if the stimulated areas do not affect the motor function of the patient, then these areas should be removed as tumor-infiltrated parenchyma [30]. The use of DES in the area that the surgeon wishes to resect enables tumor removal with a lower risk of neurological damage and favorable preservation of the important functions of each individual patient [3132]. However, awake surgery is associated with several critical features that require the cooperation of the patient and that are necessary to provide the patient with comfort. Moreover, this type of surgery requires an additional person to monitor the patient, and the surgery itself may induce a seizure. In the present study, awake surgery was not used.
Seizures are a presenting symptom in approximately 30–50% of brain tumor patients [33]. Traditionally, the management of tumor-related epilepsy has focused on tumor resection, and the seizure activity usually improves after tumor resection, particularly for meningiomas. However, recent studies have shown an epileptogenic zone that induces tumor-related epilepsy [343536]. Furthermore, tumors can cause cortical dysgenesis, gliosis, and hippocampal sclerosis, and activity in the epileptogenic zone may also lead to seizures after surgical resection of the tumor [3738]. Therefore, simple resection of the tumor mass may not be sufficient to control tumor-related epilepsy. In the present study, eight patients presented with seizures as a preoperative symptom, and three of them continued to experience seizures after the tumor resection. Thus, this is a limitation when a tumor is removed from the PMC along with surrounding brain tissue, as the epileptogenic zone may remain, and the seizures may not remit.
The present study has several limitations that should be noted. First, the present sample only included 25 patients; thus, it would be difficult to generalize the conclusions of this study to a larger population. Future large-scale studies will be necessary to confirm the present findings. Second, the present study included patients with a diverse set of pathologies, and the surgical tactics for each type of pathology typically require slightly different methods and techniques. Third, because this was a retrospective analysis, there may be some inherent bias. However, as a first analysis, this study provides several important findings regarding the resection of non-glial tumors from the PMC.
The present findings suggest that a direct surgical approach for the resection of non-glial tumors in the PMC may be safe if brain injury minimized through the use of sulcal dissection and fine tumor manipulation. Although most of the patients' neurological symptoms in the present study showed favorable outcomes, seizure activity could not be controlled. These findings indicate that a meticulous resection can be attempted in the case of a non-glial tumor in the PMC.
Figures and Tables
 | Fig. 1Surgical approach for a metastatic tumor using sulcal dissection. The tumor was located in the deep subcortex in the primary motor area (A), but it located close to the precentral sulcus (arrows). In the surgical view (B), the precentral sulcus (arrows) was fully dissected and opened into the tumor (arrowheads). The tumor was totally removed with the usual microsurgical techniques (C), and the preoperative motor weakness was completely resolved. |
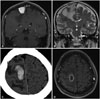 | Fig. 2Radiological images of a postoperative hematoma. A well-enhanced extra-axial mass with extensive edema was found on the right middle parasagittal region (A and B). Postoperative hematoma was developed (C). The motor symptoms were aggravated plegia of the left ankle and a subtle weakness of the arm. In a magnetic resonance imaging scan, a rim-enhancing mass was found at the hematoma area 2 months after the surgery (D). The abscess was drained. |
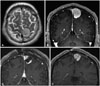 | Fig. 3Partial resection of a tumor with persistent seizures. The meningioma partially occluded the left superior sagittal sinus and showed adhesion to the motor cortex (A and B). The tumor was intentionally left at the adhesive portion of sagittal sinus and motor cortex (C). After the operation, Cyberknife radiosurgery was done with 210 cGy, but the tumor slightly increased in size (D), and the seizures remained for the following 3 years. |
Table 1
Characteristics of the cases

References
1. Ostrý S, Netuka D, Beneš V. Rolandic area meningioma resection controlled and guided by intraoperative cortical mapping. Acta Neurochir (Wien). 2012; 154:843–853.

2. Lee JJ, Kim YI, Hong JT, Sung JH, Lee SW, Yang SH. Intraoperative monitoring of motor-evoked potentials for supratentorial tumor surgery. J Korean Neurosurg Soc. 2014; 56:98–102.

3. Prabhu SS, Gasco J, Tummala S, Weinberg JS, Rao G. Intraoperative magnetic resonance imaging-guided tractography with integrated monopolar subcortical functional mapping for resection of brain tumors. Clinical article. J Neurosurg. 2011; 114:719–726.

4. Southwell DG, Hervey-Jumper SL, Perry DW, Berger MS. Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J Neurosurg. 2015; 11. 6. [Epub]. DOI: 10.3171/2015.5.JNS142833.

5. Hervey-Jumper SL, Li J, Lau D, et al. Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J Neurosurg. 2015; 123:325–339.

6. Daglioglu E, Ergungor F, Polat E, Nacar O. Microsurgical resection of supratentorial cerebral cavernomas. Turk Neurosurg. 2010; 20:348–352.
7. Kim BW, Lee JW, Huh SK, Lee KC. Clinical analysis of microsurgery for brainstem cavernous malformations: surgical indications, optimal approaches, and clinical outcomes. Korean J Cerebrovasc Surg. 2010; 12:169–176.
8. Cohen BA, Knopp EA, Rusinek H, Liu S, Gonen O. Brain compression without global neuronal loss in meningiomas: whole-brain proton MR spectroscopy report of 2 cases. AJNR Am J Neuroradiol. 2005; 26:2178–2182.
9. Weil RJ, Lonser RR. Selective excision of metastatic brain tumors originating in the motor cortex with preservation of function. J Clin Oncol. 2005; 23:1209–1217.

10. Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997; 120(Pt 1):141–157.

11. Kido DK, LeMay M, Levinson AW, Benson WE. Computed tomographic localization of the precentral gyrus. Radiology. 1980; 135:373–377.

12. Naidich TP, Valavanis AG, Kubik S. Anatomic relationships along the low-middle convexity: Part I--Normal specimens and magnetic resonance imaging. Neurosurgery. 1995; 36:517–532.

13. Yoo H, Kim YZ, Nam BH, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009; 110:730–736.

14. Stortebecker TP. Metastatic tumors of the brain from a neurosurgical point of view; a follow-up study of 158 cases. J Neurosurg. 1954; 11:84–111.
15. Kim SH, Jung S, Kang SS, et al. Evaluation of the postoperative motor function for metastatic brain tumors around the motor cortex. J Korean Neurosurg Soc. 2001; 30:Suppl 1. S25–S29.
16. Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry. 2001; 71:188–192.

17. Raychaudhuri R, Batjer HH, Awad IA. Intracranial cavernous angioma: a practical review of clinical and biological aspects. Surg Neurol. 2005; 63:319–328. discussion 328.

18. Jellinger K. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev. 1986; 9:177–216.

19. McCormick WF. The pathology of vascular ("arteriovenous") malformations. J Neurosurg. 1966; 24:807–816.

20. Robinson JR Jr, Awad IA, Magdinec M, Paranandi L. Factors predisposing to clinical disability in patients with cavernous malformations of the brain. Neurosurgery. 1993; 32:730–735. discussion 735-6.

21. Zimmerman RS, Spetzler RF, Lee KS, Zabramski JM, Hargraves RW. Cavernous malformations of the brain stem. J Neurosurg. 1991; 75:32–39.

22. Kikuta K, Nozaki K, Takahashi JA, Miyamoto S, Kikuchi H, Hashimoto N. Postoperative evaluation of microsurgical resection for cavernous malformations of the brainstem. J Neurosurg. 2004; 101:607–612.

23. Commins DL, Atkinson RD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus. 2007; 23:E3.

24. International Agency for Research on Cancer. Meningiomas. In : Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. 4th ed. Geneva: World Health Organization;2007.
25. Shinoura N, Suzuki Y, Yamada R, Kodama T, Takahashi M, Yagi K. Restored activation of primary motor area from motor reorganization and improved motor function after brain tumor resection. AJNR Am J Neuroradiol. 2006; 27:1275–1282.
26. Suzuki A, Yasui N. Intraoperative localization of the central sulcus by cortical somatosensory evoked potentials in brain tumor. Case report. J Neurosurg. 1992; 76:867–870.

27. Cedzich C, Taniguchi M, Schäfer S, Schramm J. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996; 38:962–970.

28. Tamura M, Muragaki Y, Saito T. Strategy of surgical resection for glioma based on intraoperative functional mapping and monitoring. Neurol Med Chir (Tokyo). 2015; 55:383–398.

29. Krieg SM, Schäffner M, Shiban E, et al. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions: clinical article. J Neurosurg. 2013; 118:1269–1278.

31. Duffau H. Awake mapping of the brain connectome in glioma surgery: concept is stronger than technology. Eur J Surg Oncol. 2015; 41:1261–1263.

32. Duffau H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol. 2015; 11:255–265.

33. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007; 6:421–430.

34. Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012; 70:921–928. discussion 928.

35. Mittal S, Barkmeier D, Hua J. Intracranial EEG analysis in tumor-related epilepsy: evidence of distant epileptic abnormalities. Clin Neurophysiol. 2016; 127:238–244.

36. Seo DW, Hong SB. Epileptogenic foci on subdural recording in intractable epilepsy patients with temporal dysembryoplastic neuroepithelial tumor. J Korean Med Sci. 2003; 18:559–565.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download