Abstract
Central neurocytoma (CN) is a rare, benign brain tumor often located in the lateral ventricles. CN may cause obstructive hydrocephalus and manifest as signs of increased intracranial pressure. The goal of treatment for CN is a gross total resection (GTR), which often yields excellent prognosis with a very high rate of tumor control and survival. Adjuvant radiosurgery and radiotherapy may be considered to improve tumor control when GTR cannot be achieved. Chemotherapy is also not considered a primary treatment, but has been used as a salvage therapy. The radiological features of CN are indistinguishable from those of other brain tumors; therefore, many histological markers, such as synaptophysin, can be very useful for diagnosing CNs. Furthermore, the MIB-1 Labeling Index seems to be correlated with the prognosis of CN. We also discuss oncogenes associated with these elusive tumors. Further studies may improve our ability to accurately diagnose CNs and to design the optimal treatment regimens for patients with CNs.
Central neurocytoma (CN) was first described in the 1980’s by Hassoun et al. [1] who studied two patients with intraventricular tumors using electron microscopy. CN is a benign tumor of the central nervous system that is classified as a grade II tumor by the World Health Organization (WHO) [23]. A combination of treatments, such as surgery with adjuvant ra-diation, can be considered for CN despite its good prognosis [234]. Because of the tumor’s rarity and its elusive nature, only a limited number of studies, case reports, and reviews have been published on CN.
CN remains relatively rare, comprising about 0.1–0.5% of all brain tumors [5678]. CNs are most prevalent among young adults, and nearly 25% of all cases involve individuals in their thirties [49]. The age of affected individuals ranges from 8 days old to 67 years old with an overall median age of 34 years [610]. There is no correlation between gender and the incidence of CN [791112]. Some studies have indicated higher incidences of CNs in Korea, India, and Japan, which is possibly attributed to genetic differences among racial groups that make certain individuals more prone to CNs than others [81013141516171819202122232425262728]. The higher incidence in these Asian countries, make this tumor an important consideration when dealing with intraventricular tumors in these populations.
CNs are neurocytomas located within the ventricles. Most CNs are found in the anterior half of the lateral ventricle, although some have reported to be found in the third and fourth ventricles [293031]. The tumor is also usually attached to the septum pellucidum near the foramen of Monroe [3233]. The cellular origination of CN is unclear; however, various authors have suggested CN may develop from neuronal cells, neuronal progenitor cells, neuronal stem cells, and multipotent precursor cells [112129343536].
Neurocytomas can also occur extraventricularly, along the spinal cord or the brain parenchyma [10]. Extraventricular neurocytomas (EVN) have been found in the cerebral hemi-spheres, spinal cord, brainstem, thalamus, pons, amygdala, pineal gland, retina, and cerebellum [5103738394041424344454647484950]. EVN is also classified as a grade II tumor [3]. However, EVN has a wider morphological spectrum and a higher degree of either focal or diffuse ganglionic differentiation than CN [61038515253].
CN may increase the intracranial pressure by obstructing the interventricular foramen, which can lead to hydrocephalus [3254]. Patients may also experience nausea, vomiting, headache, seizures, decreased consciousness, weakness, and memory or vision problems [47303854555657]. In rare cases, intraventricular hemorrhage may also occur [58]. Patients with EVN present with similar symptoms, in addition to weakness and numbness in the limbs [20425960]. These symptoms are typically present for approximately 3–6 months, although the duration of symptoms can vary from a few days to many years [29305561]. The duration seems to be mostly related to tumor location, and does not seem to be correlated to the aggressiveness of the tumor [4].
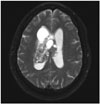
CN can appear as a dense mass in computerized tomography (CT) scans indicating calcifications, which occur in up to 50% of all cases, and present a patchy and coarse appearances (Fig. 1) [73033]. The tumor can also be heterogeneous because of the hypodense areas related to cystic degeneration [431]. In contrast-enhanced CT scans, CNs have mild to moderate enhancements [3262]. Furthermore, CNs appear slightly hypo-intense to iso-intense on T1-weighted magnetic reso-nance imaging (MRI) (Fig. 2), and iso-intense to hyper-intense on T2-weighted MRI (Fig. 3) [429313258]. In both T1- and T2-weighted MRI, hypointensity can indicate the presence of a hemorrhage, cyst, or calcification [31]. Typically, after a contrast agent is injected, moderate enhancement is seen on MRI for CN (Fig. 4) [295863]. Unfortunately, there is no estab-lished criterion to distinguish between CN and other tumors such as oligodendrogliomas on CT scans and MRI [41164].
Surgical management with a gross-total resection (GTR) is currently the gold standard treatment for CNs, which often has excellent prognosis and minimizes the chances of CN recurrence [65]. GTR is achieved in nearly 30–50% of all CN patients. In an analysis of 310 patients with CN who underwent a GTR, there was a 99% five-year survival rate [14546566]. In comparison, individuals who had surgery with only subtotal resection (STR) had an 86% five-year survival rate. STR of CN increases the rate of recurrence and decreases the rate of survival [65]. A recent multi-center study found that in 71 patients with CN, those with STR had a 3.8-fold higher risk of recurrence and adverse outcomes compared to patients with GTR [12]. For patients with STR, adjuvant radiotherapy was administered in this study.
Radiotherapy and radiosurgery are non-invasive adjuvant treatments, but the toxicities from radiation are still being weighed against the benefits of tumor control [6567]. Because CNs usually have excellent prognosis when GTR is achieved, radiation is not always indicated [255455]. Radiotherapy and radiosurgery have been adopted as an adjuvant treatment when GTR cannot be achieved, the patient is inoperable, or the tumor is aggressive [1261].
A recent report suggests that fractionated radiotherapy (FRT) after STR had a statistically significant higher tumor control rate and improved survival in adults [1168]. A higher 5-year progression free survival has also been shown for patients who received adjuvant FRT after STR (67%) than patients without FRT (53%) [69].
While FRT delivers multiple fractions of radiation in lower dosage, stereotactic radiosurgery (SRS) administers one higher dose (9 to 25 Gy) of radiation in 1-5 fractions. The first literature on the use of SRS for CN was published by Schild et al. [54]. SRS has been suggested to be potentially favorable to FRT. Although not statistically significant, Patel et al. [11] also reported that adjuvant SRS for patients with STRs demonstrated a 100% tumor control rate compared to an 87% tumor control rate for patients with adjuvant FRT [54].
Garcia et al. [70] also reported a higher tumor control rate of 93% with SRS versus 88% with FRT. The relative risk (RR) of SRS to FRT for recurrence was 0.57 less (95% CI: 0.21–1.57; log-rank p=0.85), and the RR for mortality was 0.23 less (95% CI: 0.05–1.05; log-rank p=0.22), although statistically insignificant. Lower complication was noted for patients with SRS, although distant tumor recurrence was slightly higher in patients who received SRS than those who received FRT. SRS is suggested to be at least as effective as FRT in achieving tumor control.
Schild et al. [54] reported a 5-year survival rate for patients who received FRT or SRS after surgical resection of 88%, while the 5-year survival rate for patients without adjuvant radiation was only 71%. Imber et al. [69] also found significantly improved survival rates when adjuvant radiotherapy is administered following STR. Patients with STR and FRT had a 67% 5-year survival rate, while patients with STR only had a 53% survival rate. Additionally, Kim et al. [71] reported that patients with STR had a lower recurrence rate after adjuvant therapy of FRT or SRS (1 of 12 patients) when compared to patients without adjuvant radiation (3 of 12 patients), although the difference was statistically insignificant. Overall, adjuvant therapy following incomplete resection of CNs appears to result in better tumor control.
Complications can arise from radiation therapy. A single institutional study found that 4 out of 7 patients who received FRT exhibited complications, such as white matter degradation or radiation necrosis, although FRT was effective in achieving local tumor control [71]. Chen et al. [72] found that among 60 patients treated with radiation therapy, 28 patients exhibited grade I neurotoxicity which resulted in short-term memory impairment and motor deficit. Seven patients displayed grade II neurotoxicity, while three patients had grade III neurotoxicity [72]. The associated symptoms included cognitive disturbance, hemianopsia, seizure, and involuntary movement [72].
Although chemotherapy is not a primary treatment modality for CN, chemotherapy has been used as an adjuvant or salvage therapy for recurrent CNs or inoperable patients [113173]. There are no studies using chemotherapy as a primary form of treatment for CN, nor a comparison of the efficacy between radiotherapy and chemotherapy as an adjuvant treatment [411737475]. Only a few case reports noted partial tumor regression following chemotherapy, and only one study reported a child with a complete response using a combination of topotecan, carboplatin, and phosphamide in three cycles [475767778].
CN has been relatively difficult to diagnose because of its histopathological similarity to other brain tumors, such as oligodendrogliomas and ependymomas [41164]. Light microscopy is ineffective in identifying CNs [97980]. Generally, immunohistochemistry is performed for the diagnosis of CN [92979].
The histology of CN can vary throughout a single specimen and is typically benign (Fig. 5) [10]. The tumor cells create a "honeycomb pattern," and appear small and round with scant cytoplasm and stippled chromatin [410243132]. Since these characteristics are similar to the appearance of oligodendrogliomas, there is a potential opportunity for misdiagnosis [41011243132]. Likewise, the pathological features of ependymo-mas are similar to the perivascular rosette or straight line cell arrangements that are also seen in CN [10]. Therefore, multiple immunohistochemical markers are helpful in differentiating CNs from other tumors.
Although less commonly used, electron microscopy can be another helpful tool in diagnosing patients with CNs by looking for parallel arrays of microtubules with dense-core neurosecretory granules and clear vesicles [7212436].
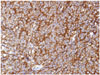
Synaptophysin is one of the major molecular markers for CN [10]. Positive staining for synaptophysin, a transmembrane glycoprotein present in presynaptic vesicles of neurons, is a strong indicator for neuronal cells and its neoplasms (Fig. 6). Synaptophysin staining is usually found in the fibrillary and perivascular areas of CN [98182].
In addition to positivity for synaptophysin, negativity for neuron specific enolase (NSE) and vimentin has been reported to suggest CN over oligodendroglioma and clear cell ependymoma (Table 1) [4]. NSE is a glycolytic enzyme located in the cytoplasm of neurons [83]. Although it is present in CN, it lacks neuronal specificity and has been reported to be present in non-neuronal neoplasms [924]. Vimentin is an intermediate filament protein found in glial cells, and is usually present in oligodendrogliomas and clear cell ependymomas, but absent in CN [8485].
Epithelial membrane antigen (EMA) is another protein that differentiates CN from oligodendroglioma and ependymoma. EMA, which is normally expressed in epithelial cells, is present in ependymal cells in the central nervous system, as well as in ependymomas [86]. Furthermore, EMA positivity has also been linked with other glial tumors such as glioblastoma, astrocytoma, and oligodendroglioma [8687]. Thus positivity of EMA can suggest ependymoma and oligodendroglioma over CN [4].
Neuronal nuclei (NeuN) is present in the nuclei and perinuclear cytoplasm of post-mitotic neurons in the central nervous system. Positive staining for NeuN suggests the neuronal nature of neoplasms and is considered to be a reliable marker for clear cell neoplasms of the central nervous system, which include CN, oligodendroglioma, and clear cell ependymoma [48889]. It has also been reported that positive staining for NeuN correlates with a lower proliferation index [490].
Glial fibrilllary acidic protein (GFAP), which is detected in glial cell tumors, is usually absent in CN. It is the most abundant intermediate filament protein in astrocytes, and is usually present in astrocytes that infiltrate or surround CN [4215152539192]. Cases of CN with GFAP positivity suggest glial differentiation of bipotential (astrocytic and neuronal) precursor cells, and also correlates with a more malignant disease course [93].
Neurofilament (NF), which exists as intermediate filaments in neurons, is largely absent in CN. This suggests that the full differentiation of CN cells to developed neurons is rare [1234]. Vasiljevic et al. [12] reported that positivity in NF is a key diagnostic difference between CN and pineal parenchymal tumor.
Oligodendrocyte transcription factor 2 (Olig2), which is a transcription factor that regulates oligodendroglial differentiation, is also generally absent in CN. Olig2 can be used as a diagnostic marker for oligodendroglioma, and positivity for Olig2 suggests oligodendroglioma over CN [9495]. This also argues the rarity of CN cells undergoing glial differentiation.
Chromagranin A (chrA) is a neuroendocrine protein located on secretory vesicles of neurons. ChrA is generally absent in CN, but cases of positivity have been reported [969798]. Peng et al. [96] suggested that the positivity of chromogranin A may be due to the presence of ganglion cells in CN. Overall, ChrA is not a reliable marker for the diagnosis of CNs; however, ChrA positivity in some CNs may provide an insight to the cellular and developmental origins of CN [98].
The MIB-1 Labeling Index (MIB-1 LI) is an important prognostic tool for CN (Fig. 7). In 2015, Imber et al. [69] measured progression-free survival (PFS) by Kaplan-Meier and Cox proportional hazards methods, and found that the two year PFS was 48% for MIB-1 LI >4%, and 90% for MIB-1 LI <4%. Similarly, CN with MIB-1 LI <2% had a 10-year survival rate of 90%, compared to MIB-1 LI >2%, which had a 10-year survival rate of 63%.
MIB-1 LI has also been reported to be an indicator of tumor relapse. It has been reported that CN with MIB-1 LI >2% had a 63% chance of recurrence; whereas CN with MIB LI <2% had only a 22% chance of recurrence over a 150-month period [499]. Furthermore, Chen et al. [93] found that out of the nine patients presented in the study, the four that experienced tumor recurrence or death from continuous tumor growth or surgical complications had MIB-1 LI >2%, suggesting that an MIB-1 LI >2% may indicate a more aggressive disease course.
MIB-1 LI may also be used to determine the tumor grade. Sharma et al. [18] reported that the only proliferation marker that correlated with CN atypia is the MIB-1 LI. Söylemezoglu et al. [52] found that a MIB-1 LI >2% correlated with microvascular proliferation and suggested that a CN with MIB-1 LI >2% should be termed ‘atypical’ [4]. Atypical CN are also known to spread through the cerebrospinal fluid and metastasize in the ventricles or the spinal cord [100101102103].
Many types of genetic mutations have been associated with CNs. N-myc proto-oncogene (N-Myc), which is an oncogene associated with the development of other cancers such as neuroblastoma and medulloblastoma, is overexpressed in CN [34104105106]. The overexpression of N-Myc in neuroblastoma seems to indicate a poorer prognosis [34107]. N-Myc is required for neural proliferation, but inhibits complete neural differentiation of neuronal progenitor cells [34108]. The levels of N-Myc are inversely related to the tumor suppressor gene encoding Myc box-dependent-interacting (BIN-1) protein, which has been found to be significantly underexpressed in neurocytomas, as well as other tumors [34105]. This suggests that CN may contain a mutation somewhere in the pathway that includes both N-Myc and BIN-1, and that the respective overexpression and underexpression of N-Myc and BIN-1 may play a large role in CN tumorigenesis [34105].
Phosphatase and tensin homolog (PTEN) gene is a tumor suppressor gene also found to be overexpressed in CN [105]. Musatov et al. [109] showed that PTEN overexpression inhibits neural differentiation in PC12 cells by phosphoinositide 3-kinase and mitogen-activated protein kinase pathways [34]. Thus, PTEN and N-Myc overexpression together could po-tentially warrant the lack of full neuronal differentiation frequently seen in CN.
In addition, Sim et al. [35] found that insulin-like growth factor 2 was overexpressed in CN cells compared to cells in the ventricular zone, and may play a key role in the proliferation of neurocytoma, similar to its role in the proliferation of glioblastoma multiforme [34110].
Similarly, platelet-derived growth factor D (PDGF-D) and neureglin 2 (NRG-2) were found to be overexpressed in CN. PDGF-D overexpression has been found to be involved with the maturation of certain tumors [34111], whereas NRG-2 overexpression has been linked to the proliferation of neuroblasts, as well as the aggressiveness in breast carcinoma [34112113]. Overall, PDGF-D and NRG-2 can also offer an explanation for the tumorigenesis of neuronal progenitor cells [34].
CN is a benign tumor of the CNS that has an excellent prognosis. Surgery with gross total resection is the most preferable, correlated with the best long-term survival rates and local tumor control. Adjuvant radiotherapy may be considered for residual CN following STR, large CN size, or CNs near inoperable regions. Radiotherapy or chemotherapy the primary treatment for CNs has not been thoroughly examined.
Many histological markers are available for the diagnosis of CN, although some markers are also sensitive to other tumors. The MIB-1 LI is currently the most accurate tool to determine prognosis, tumor relapse, and tumor grade. Further molecular and genetic studies may offer insights into other immunohistochemical methods for improved diagnostic accuracy.
Figures and Tables
Fig. 1
Axial CT demonstrating a large hypodense central neurocytoma. Moderate, heterogenous hyperdensities are consistent with calcifications.
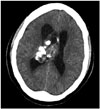
Fig. 2
Axial T1-weighted MRI showing a large isointense CN in the lateral ventricles consistent with CN. CN, central neurocytoma.
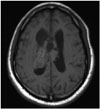
Fig. 4
Axial T1-weighted MRI with contrast showing a large isointense central neurocytoma with moderate enhancement.
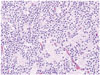
Fig. 5
H&E stained slide of central neurocytoma showing a proliferation of small round, fairly uniform nuclei with perinuclear haloes. H&E, hematoxyin eosin.
Acknowledgments
Seung Jin Lee (first author) was funded by the American Academy of Neurology Medical Student Summer Research Scholarship. Isaac Yang (senior author) was partially supported by a Visionary Fund Grant, a UCLA Stein Oppenheimer grant, and the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research UCLA Scholars in Translational Medicine Program Award.
References
1. Hassoun J, Gambarelli D, Grisoli F, et al. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982; 56:151–156.
2. Yang I, Ung N, Chung LK, et al. Clinical manifestations of central neurocytoma. Neurosurg Clin N Am. 2015; 26:5–10.

3. Louis DN, Ohgaki H, Wiestler OD. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.

4. Choudhari KA, Kaliaperumal C, Jain A, et al. Central neurocytoma: a multi-disciplinary review. Br J Neurosurg. 2009; 23:585–595.

5. Coca S, Moreno M, Martos JA, Rodriguez J, Barcena A, Vaquero J. Neurocytoma of spinal cord. Acta Neuropathol. 1994; 87:537–540.

6. Figarella-Branger D, Soylemezoglu F, Kleihues P, Hassoun J. Central neurocytoma. In : Kleihues P, Cavenee WK, editors. Pathology and genetics of tumors of the nervous system. Lyon: IARC Press;2000. p. 107–109.
7. Hassoun J, Söylemezoglu F, Gambarelli D, Figarella-Branger D, von Ammon K, Kleihues P. Central neurocytoma: a synopsis of clinical and histological features. Brain Pathol. 1993; 3:297–306.

8. Kim DG, Chi JG, Park SH, et al. Intraventricular neurocytoma: clinicopathological analysis of seven cases. J Neurosurg. 1992; 76:759–765.

9. Maiuri F, Spaziante R, De Caro ML, Cappabianca P, Giamundo A, Iaconetta G. Central neurocytoma: clinico-pathological study of 5 cases and review of the literature. Clin Neurol Neurosurg. 1995; 97:219–228.

10. Sharma MC, Deb P, Sharma S, Sarkar C. Neurocytoma: a comprehensive review. Neurosurg Rev. 2006; 29:270–285. discussion 285.

11. Patel DM, Schmidt RF, Liu JK. Update on the diagnosis, pathogenesis, and treatment strategies for central neurocytoma. J Clin Neurosci. 2013; 20:1193–1199.

12. Vasiljevic A, François P, Loundou A, et al. Prognostic factors in central neurocytomas: a multicenter study of 71 cases. Am J Surg Pathol. 2012; 36:220–227.
13. Kim DG, Kim JS, Chi JG, et al. Central neurocytoma: proliferative potential and biological behavior. J Neurosurg. 1996; 84:742–747.

14. Kim DG, Paek SH, Kim IH, et al. Central neurocytoma: The role of radiation therapy and long-term outcome. Cancer. 1997; 79:1995–2002.
15. Kim DH, Suh YL. Pseudopapillary neurocytoma of temporal lobe with glial differentiation. Acta Neuropathol. 1997; 94:187–191.

16. Kim DG, Choe WJ, Chang KH. In vivo proton magnetic resonance spectroscopy of central neurocytomas. Neurosurgery. 2000; 46:329–333. discussion 333-4.

17. Kulkarni V, Rajshekhar V, Haran RP, Chandi SM. Long-term outcome in patients with central neurocytoma following stereotactic biopsy and radiation therapy. Br J Neurosurg. 2002; 16:126–132.

18. Sharma MC, Rathore A, Karak AK, Sarkar C. A study of proliferative markers in central neurocytoma. Pathology. 1998; 30:355–359.

19. Sharma MC, Sarkar C, Karak AK, Gaikwad S, Mahapatra AK, Mehta VS. Intraventricular neurocytoma: a clinicopathological study of 20 cases with review of the literature. J Clin Neurosci. 1999; 6:319–323.

20. Sharma S, Sarkar C, Gaikwad S, Suri A, Sharma MC. Primary neurocytoma of the spinal cord: a case report and review of literature. J Neurooncol. 2005; 74:47–52.

21. Ishiuchi S, Tamura M. Central neurocytoma: an immunohistochemical, ultrastructural and cell culture study. Acta Neuropathol. 1997; 94:425–435.

22. Ishiuchi S, Nakazato Y, Iino M, Ozawa S, Tamura M, Ohye C. In vitro neuronal and glial production and differentiation of human central neurocytoma cells. J Neurosci Res. 1998; 51:526–535.

23. Kawashima M, Suzuki SO, Doh-ura K, Iwaki T. alpha-Synuclein is expressed in a variety of brain tumors showing neuronal differentiation. Acta Neuropathol. 2000; 99:154–160.

24. Kubota T, Hayashi M, Kawano H, et al. Central neurocytoma: immunohistochemical and ultrastructural study. Acta Neuropathol. 1991; 81:418–427.

25. Nakagawa K, Aoki Y, Sakata K, Sasaki Y, Matsutani M, Akanuma A. Radiation therapy of well-differentiated neuroblastoma and central neurocytoma. Cancer. 1993; 72:1350–1355.

26. Namiki J, Nakatsukasa M, Murase I, Yamazaki K. Central neurocytoma presenting with intratumoral hemorrhage 15 years after initial treatment by partial removal and irradiation. Neurol Med Chir (Tokyo). 1998; 38:278–282.

27. Nishio S, Takeshita I, Kaneko Y, Fukui M. Cerebral neurocytoma. A new subset of benign neuronal tumors of the cerebrum. Cancer. 1992; 70:529–537.

28. Utsunomiya A, Uenohara H, Suzuki S, et al. [A case of anaplastic astrocytoma arising 8 years after initial treatment by partial resection and irradiation for central neurocytoma]. No To Shinkei. 2001; 53:747–751.
30. Chandrashekhar TN, Mahadevan A, Vani S, et al. Pathological spectrum of neuronal/glioneuronal tumors from a tertiary referral neurological Institute. Neuropathology. 2012; 32:1–12.

31. Schmidt MH, Gottfried ON, von Koch CS, Chang SM, McDermott MW. Central neurocytoma: a review. J Neurooncol. 2004; 66:377–384.

32. Shin JH, Lee HK, Khang SK, et al. Neuronal tumors of the central nervous system: radiologic findings and pathologic correlation. Radiographics. 2002; 22:1177–1189.

33. Goergen SK, Gonzales MF, McLean CA. Interventricular neurocytoma: radiologic features and review of the literature. Radiology. 1992; 182:787–792.

34. Kane AJ, Sughrue ME, Rutkowski MJ, Tihan T, Parsa AT. The molecular pathology of central neurocytomas. J Clin Neurosci. 2011; 18:1–6.

35. Sim FJ, Keyoung HM, Goldman JE, et al. Neurocytoma is a tumor of adult neuronal progenitor cells. J Neurosci. 2006; 26:12544–12555.

36. Tsuchida T, Matsumoto M, Shirayama Y, Imahori T, Kasai H, Kawamoto K. Neuronal and glial characteristics of central neurocytoma: electron microscopical analysis of two cases. Acta Neuropathol. 1996; 91:573–577.

37. Giangaspero F, Cenacchi G, Losi L, Cerasoli S, Bisceglia M, Burger PC. Extraventricular neoplasms with neurocytoma features. A clinicopathological study of 11 cases. Am J Surg Pathol. 1997; 21:206–212.
38. Brat DJ, Scheithauer BW, Eberhart CG, Burger PC. Extraventricular neurocytomas: pathologic features and clinical outcome. Am J Surg Pathol. 2001; 25:1252–1260.
39. Lenzi J, Salvati M, Raco A, Frati A, Piccirilli M, Delfini R. Central neurocytoma: a novel appraisal of a polymorphic pathology. Our experience and a review of the literature. Neurosurg Rev. 2006; 29:286–292. discussion 292.

40. Ogiwara H, Dubner S, Bigio E, Chandler J. Neurocytoma of the cerebellum. Surg Neurol Int. 2011; 2:36.

41. Louis DN, Swearingen B, Linggood RM, et al. Central nervous system neurocytoma and neuroblastoma in adults--report of eight cases. J Neurooncol. 1990; 9:231–238.

42. Tatter SB, Borges LF, Louis DN. Central neurocytomas of the cervical spinal cord. Report of two cases. J Neurosurg. 1994; 81:288–293.
44. Ng P, Soo YS, Chaseling R, O’Neil P. Intraventricular neurocytoma. Australas Radiol. 1996; 40:125–133.

45. Pal L, Santosh V, Gayathri N, et al. Neurocytoma/rhabdomyoma (myoneurocytoma) of the cerebellum. Acta Neuropathol. 1998; 95:318–323.

46. Rabinowicz AL, Abrey LE, Hinton DR, Couldwell WT. Cerebral neurocytoma: an unusual cause of refractory epilepsy. Case report and review of the literature. Epilepsia. 1995; 36:1237–1240.

48. Sgouros S, Walsh AR, Barber P. Central neurocytoma of thalamic origin. Br J Neurosurg. 1994; 8:373–376.

49. Ahmad F, Rosenblum MK, Chamyan G, Sandberg DI. Infiltrative brainstem and cerebellar neurocytoma. J Neurosurg Pediatr. 2012; 10:418–422.

50. Enam SA, Rosenblum ML, Ho KL. Neurocytoma in the cerebellum. Case report. J Neurosurg. 1997; 87:100–102.
51. Figarella-Branger D, Pellissier JF, Daumas-Duport C, et al. Central neurocytomas. Critical evaluation of a small-cell neuronal tumor. Am J Surg Pathol. 1992; 16:97–109.
52. Söylemezoglu F, Scheithauer BW, Esteve J, Kleihues P. Atypical central neurocytoma. J Neuropathol Exp Neurol. 1997; 56:551–556.

53. von Deimling A, Janzer R, Kleihues P, Wiestler OD. Patterns of differentiation in central neurocytoma. An immunohistochemical study of eleven biopsies. Acta Neuropathol. 1990; 79:473–479.
55. Ashkan K, Casey AT, D’Arrigo C, Harkness WF, Thomas DG. Benign central neurocytoma. Cancer. 2000; 89:1111–1120.

56. Agranovich AL, Ang LC, Fryer CJ. Central neurocytoma: report of 2 cases and literature review. J Neurooncol. 1993; 16:47–53.

57. Moussa R, Abadjian G, Nader M, et al. [Central neurocytoma. Four patients]. Neurochirurgie. 2004; 50:639–646.
58. Jaiswal S, Vij M, Rajput D, et al. A clinicopathological, immunohistochemical and neuroradiological study of eight patients with central neurocytoma. J Clin Neurosci. 2011; 18:334–339.

59. Martin AJ, Sharr MM, Teddy PJ, Gardner BP, Robinson SF. Neurocytoma of the thoracic spinal cord. Acta Neurochir (Wien). 2002; 144:823–828.

60. Stapleton SR, David KM, Harkness WF, Harding BN. Central neurocytoma of the cervical spinal cord. J Neurol Neurosurg Psychiatry. 1997; 63:119.

61. Leenstra JL, Rodriguez FJ, Frechette CM, et al. Central neurocytoma: management recommendations based on a 35-year experience. Int J Radiat Oncol Biol Phys. 2007; 67:1145–1154.

62. Zhang D, Wen L, Henning TD, et al. Central neurocytoma: clinical, pathological and neuroradiological findings. Clin Radiol. 2006; 61:348–357.

63. Wichmann W, Schubiger O, von Deimling A, Schenker C, Valavanis A. Neuroradiology of central neurocytoma. Neuroradiology. 1991; 33:143–148.

64. Shah T, Jayasundar R, Singh VP, Sarkar C. MRS characterization of central neurocytomas using glycine. NMR Biomed. 2011; 24:1408–1413.

66. Bertalanffy A, Roessler K, Koperek O, Gelpi E, Prayer D, Knosp E. Recurrent central neurocytomas. Cancer. 2005; 104:135–142.

67. Rades D, Fehlauer F, Lamszus K, et al. Well-differentiated neurocytoma: what is the best available treatment? Neuro Oncol. 2005; 7:77–83.
68. Rades D, Schild SE. Treatment recommendations for the various subgroups of neurocytomas. J Neurooncol. 2006; 77:305–309.

69. Imber BS, Braunstein SE, Wu FY, et al. Clinical outcome and prognostic factors for central neurocytoma: twenty year institutional experience. J Neurooncol. 2016; 126:193–200.

70. Garcia RM, Ivan ME, Oh T, Barani I, Parsa AT. Intraventricular neurocytomas: a systematic review of stereotactic radiosurgery and fractionated conventional radiotherapy for residual or recurrent tumors. Clin Neurol Neurosurg. 2014; 117:55–64.

71. Kim JW, Kim DG, Chung HT, et al. Radiosurgery for central neurocytoma: long-term outcome and failure pattern. J Neurooncol. 2013; 115:505–511.

72. Chen YD, Li WB, Feng J, Qiu XG. Long-term outcomes of adjuvant radiotherapy after surgical resection of central neurocytoma. Radiat Oncol. 2014; 9:242.

73. von Koch CS, Schmidt MH, Uyehara-Lock JH, Berger MS, Chang SM. The role of PCV chemotherapy in the treatment of central neurocytoma: illustration of a case and review of the literature. Surg Neurol. 2003; 60:560–565.

74. Buchbinder D, Danielpour M, Yong WH, Salamon N, Lasky J. Treatment of atypical central neurocytoma in a child with high dose chemotherapy and autologous stem cell rescue. J Neurooncol. 2010; 97:429–437.

75. Brandes AA, Amistà P, Gardiman M, et al. Chemotherapy in patients with recurrent and progressive central neurocytoma. Cancer. 2000; 88:169–174.

76. Sgouros S, Carey M, Aluwihare N, Barber P, Jackowski A. Central neurocytoma: a correlative clinicopathologic and radiologic analysis. Surg Neurol. 1998; 49:197–204.

77. Dodds D, Nonis J, Mehta M, Rampling R. Central neurocytoma: a clinical study of response to chemotherapy. J Neurooncol. 1997; 34:279–283.
78. Amini E, Roffidal T, Lee A, et al. Central neurocytoma responsive to topotecan, ifosfamide, carboplatin. Pediatr Blood Cancer. 2008; 51:137–140.

79. Yasargil MG, von Ammon K, von Deimling A, Valavanis A, Wichmann W, Wiestler OD. Central neurocytoma: histopathological variants and therapeutic approaches. J Neurosurg. 1992; 76:32–37.

80. Valdueza JM, Westphal M, Vortmeyer A, Muller D, Padberg B, Herrmann HD. Central neurocytoma: clinical, immunohistologic, and biologic findings of a human neuroglial progenitor tumor. Surg Neurol. 1996; 45:49–56.

81. Gould VE, Wiedenmann B, Lee I, et al. Synaptophysin expression in neuroendocrine neoplasms as determined by immunocytochemistry. Am J Pathol. 1987; 126:243–257.
82. Kolos YA, Grigoriyev IP, Korzhevskyi DE. [A synaptic marker synaptophysin]. Morfologiia. 2015; 147:78–82.
83. Schmidt FM, Mergl R, Stach B, Jahn I, Gertz HJ, Schönknecht P. Elevated levels of cerebrospinal fluid neuron-specific enolase (NSE) in Alzheimer’s disease. Neurosci Lett. 2014; 570:81–85.

84. Cochard P, Paulin D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J Neurosci. 1984; 4:2080–2094.

85. Tezcan O, Gündüz U. Vimentin silencing effect on invasive and migration characteristics of doxorubicin resistant MCF-7 cells. Biomed Pharmacother. 2014; 68:357–364.

86. Koperek O, Gelpi E, Birner P, Haberler C, Budka H, Hainfellner JA. Value and limits of immunohistochemistry in differential diagnosis of clear cell primary brain tumors. Acta Neuropathol. 2004; 108:24–30.

87. Hasselblatt M, Paulus W. Sensitivity and specificity of epithelial membrane antigen staining patterns in ependymomas. Acta Neuropathol. 2003; 106:385–388.

88. Gusel’nikova VV, Korzhevskiy DE. NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Naturae. 2015; 7:42–47.

89. Duan W, Zhang YP, Hou Z, et al. Novel insights into NeuN: from neuronal marker to splicing regulator. Mol Neurobiol. 2016; 53:1637–1647.

90. Englund C, Alvord EC Jr, Folkerth RD, et al. NeuN expression correlates with reduced mitotic index of neoplastic cells in central neurocytomas. Neuropathol Appl Neurobiol. 2005; 31:429–438.

91. Hessler RB, Lopes MB, Frankfurter A, Reidy J, VandenBerg SR. Cytoskeletal immunohistochemistry of central neurocytomas. Am J Surg Pathol. 1992; 16:1031–1038.

92. Bai X, Saab AS, Huang W, Hoberg IK, Kirchhoff F, Scheller A. Genetic background affects human glial fibrillary acidic protein promoter activity. PLoS One. 2013; 8:e66873.

93. Chen CL, Shen CC, Wang J, Lu CH, Lee HT. Central neurocytoma: a clinical, radiological and pathological study of nine cases. Clin Neurol Neurosurg. 2008; 110:129–136.

94. Han L, Niu H, Wang J, et al. Extraventricular neurocytoma in pediatric populations: a case report and review of the literature. Oncol Lett. 2013; 6:1397–1405.

95. Yokoo H, Nobusawa S, Takebayashi H, et al. Anti-human Olig2 antibody as a useful immunohistochemical marker of normal oligodendrocytes and gliomas. Am J Pathol. 2004; 164:1717–1725.

96. Peng P, Chen F, Zhou D, Liu H, Li J. Neurocytoma of the pituitary gland: a case report and literature review. Biomed Rep. 2015; 3:301–303.

97. Moriguchi S, Yamashita A, Marutsuka K, et al. Atypical extraventricular neurocytoma. Pathol Int. 2006; 56:25–29.

98. You H, Kim YI, Im SY, et al. Immunohistochemical study of central neurocytoma, subependymoma, and subependymal giant cell astrocytoma. J Neurooncol. 2005; 74:1–8.

99. Mackenzie IR. Central neurocytoma: histologic atypia, proliferation potential, and clinical outcome. Cancer. 1999; 85:1606–1610.
100. Vajrala G, Jain PK, Surana S, Madigubba S, Immaneni SR, Panigrahi MK. Atypical neurocytoma: dilemma in diagnosis and management. Surg Neurol Int. 2014; 5:183.

101. Cook DJ, Christie SD, Macaulay RJ, Rheaume DE, Holness RO. Fourth ventricular neurocytoma: case report and review of the literature. Can J Neurol Sci. 2004; 31:558–564.

102. Eng DY, DeMonte F, Ginsberg L, Fuller GN, Jaeckle K. Craniospinal dissemination of central neurocytoma. Report of two cases. J Neurosurg. 1997; 86:547–552.
103. Mozes P, Szanto E, Tiszlavicz L, et al. Clinical course of central neurocytoma with malignant transformation-an indication for craniospinal irradiation. Pathol Oncol Res. 2014; 20:319–325.

104. Kleihues P, Sobin LH. World Health Organization classification of tumors. Cancer. 2000; 88:2887.

105. Korshunov A, Sycheva R, Golanov A. Recurrent cytogenetic aberrations in central neurocytomas and their biological relevance. Acta Neuropathol. 2007; 113:303–312.

106. Konovalov AN, Mariashev SA, Golanov AV, et al. [Results of surgical treatment in patients with neurocytomas of the brain]. Zh Vopr Neirokhir Im N N Burdenko. 2006; (4):5–10. discussion 10.
107. Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003; 3:203–216.

108. Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002; 16:2699–2712.

109. Musatov S, Roberts J, Brooks AI, et al. Inhibition of neuronal phenotype by PTEN in PC12 cells. Proc Natl Acad Sci U S A. 2004; 101:3627–3631.

110. Soroceanu L, Kharbanda S, Chen R, et al. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc Natl Acad Sci U S A. 2007; 104:3466–3471.

111. LaRochelle WJ, Jeffers M, Corvalan JR, et al. Platelet-derived growth factor D: tumorigenicity in mice and dysregulated expression in human cancer. Cancer Res. 2002; 62:2468–2473.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download