Abstract
Ependymomas are the most common intramedullary spinal cord tumors in adults. Although a hemorrhage within spinal ependymoma on imaging studies is not uncommon, it has rarely been reported to bea cause of acute neurological deficit. In the present report, we describe a case of a 24-year-old female patient who developed acute paraplegia as a result of hemorrhagic spinal ependymoma immediately after a cesarean delivery under spinal regional anesthesia. We review the literature of hemorrhagic spinal ependymomas presenting with acute neurological deficit and discuss the most appropriate treatment for a good neurological recovery.
Intramedullary spinal cord tumors, including ependymomas, have been rarely reported in previous literature and the treatment of these tumors has been challenging. Ependymomas are the most common intramedullary spinal cord tumors in adults and most frequently occur in children and young adults [12], accounting for 1% to 5% of all spinal tumors [2]. The typical initial symptoms of the patients with spinal ependymomas include pain, lower-extremity paresthesia, weakness, and bladder dysfunction [3].
Several reports have described a hemorrhage within spinal ependymoma on imaging studies [4]. However, the presence of hemorrhage within spinal ependymoma causing acute neurological deficit, such as paraplegia, has been rarely reported (Table 1) [156789101112]. In this report, we describe a cause of a young patient who developed acute paraplegia as a result of hemorrhagic spinal ependymoma immediately after a cesarean delivery under spinal regional anesthesia. In addition, we review the literature of hemorrhagic spinal ependymomas presenting with acute neurological deficit and discuss the most appropriate treatment for a good recovery from this neurological deterioration.
A 24-year-old female patient presented with acute paraplegia which occurred immediately after a cesarean delivery under spinal regional anesthesia. She was a healthy woman without any neurological dysfunction or any other medical history. She had no important bleeding risk factors, including medical treatment for anticoagulation, and no history of heavy lifting or trauma. In another hospital, she underwent a normal cesarean section under spinal epidural anesthesia and the whole procedure had no specific complications. However, one day after delivery, paraplegia and sensory loss below D7 dermatome did not recover. In addition, she could not feel any sense of defecation and urination. Anal tone was impaired and fecal compaction was discovered. Other symptoms included a mild fever and neck stiffness as well. She was admitted to our neurosurgical department and spine magnetic resonance image (MRI) scan was performed. Her initial spine MRI scan showed an abnormal spinal cord lesion from C2 level to D5, which was suspicious of a spinal cord swelling with a proximal syrinx or a hemorrhage formation. The lesion showed heterogeneous high signal intensity on T2-weighted MRI. On T1-weighted image, the lesion showed irregular rim enhancement (Fig. 1). In our case, the margin of enhancing lesion was poorly demarcated. Considering the patient's history, namely, the lack of symptoms prior to delivery and then the occurrence of acute neurological symptom immediately after delivery under spinal anesthesia, acute bleeding of intramedullary tumor or infection, or other autoimmune diseases were suspicious as a differential diagnosis. The patient had a mild fever with neck stiffness, however Kernig sign and Bruzinski sign were both negative in this patient. For exclusion of central nervous system infection, cerebrospinal fluid (CSF) study and laboratory study were undertaken. On CSF study, the value of white blood cell (WBC), red blood cell, protein, and glucose was 2, 0, 37, and 82, respectively. This result was within the normal range. Additionally, CSF culture was done, however it showed no growth of bacteria. On laboratory study, WBC, erythrocyte sedimentation rate, and C-reactive protein were within normal range, however the percentage of segmented neutrophil count was increased up to 86.9%. The patient's brain MRI scan was also done and no specific abnormal lesion was observed. Antibiotics and steroid therapy were alternatively prescribed each for few days to rule out the differential diagnoses. After 2 weeks of medical treatment, the patient showed neither specific improvement nor aggravation. Then, a spine MRI scan was performed to follow up the abnormal lesion and to prepare for operation (Fig. 2). The patient's MRI scan showed that the lesion slightly diminished in size. To confirm the intradural lesion, wide unilateral hemilaminectomies were performed on the left side of C7 and T1 level (Fig. 3). After opening the dura, a midline pial incision was done precisely and a gray and sticky mass was observed right below the pial incision. After the mass was partially resected, a dark hematoma was observed inside and beneath the mass. A large amount of dark hematoma could be sucked out easily. There were no specific complications during surgery. After surgery, the patient's symptoms slightly improved. She could sense pain and temperature above the knee level. Her postoperative MRI scan showed that the lesion significantly decreased (Fig. 4). 5 days after surgery, a histopathological examination confirmed the diagnosis of ependymoma (World Health Organization grade II) with hemorrhagic component. On immunohistochemistry study, glial fibrillary acidic protein was weakly positive, epithelial membrane antigen was negative and Ki-67 was 4%. To entirely remove the ependymoma, we planned the second surgery one week after the first surgery. Her preoperative spinal MRI scan for the second surgery showed that a huge amount of the abnormal lesion decreased. The central portion, which was the hematoma, significantly diminished and the enhancing portion, which was an ependymoma, was partially removed as well (Fig. 5). Unilateral hemilaminectomies were extended from C6 level to T2 level. Although the mass was very sticky and a severe adhesion was noted, the tumor could be subtotally removed with minimal damage to the spinal cord. Interaoperative monitoring was performed and it did not show significant changes during surgery. After the second surgery, the patient's postoperative spinal MRI scan showed that most of the enhancing mass was removed and that only a very thin layered enhancing portion remained at C7 level (Fig. 6). In conclusion, the tumor was subtotally removed. The patient's sense of temperature improved above the thigh level. However, her motor functions and cauda equine syndrome did not improve. Her Karnofsky performance scale score was 50. The patient was under the rehabilitation therapy and then was discharged without any other complications. At present, radio-therapy for the remaining lesion is planned, according to previous reports which insisted benefits in progression free survival of adjuvant radiotherapy in the patients with spinal ependymoma [5111415161718].
In the spinal cord, ependymomas are the most common neuroepithelial tumors, accounting for 50% to 60% of all adult spinal cord tumors [16]. Ependymomas are usually well circumscribed with a smooth and regular margin [15]. The ultimate goal of the treatment of spinal cord ependymomas is the progression-free survival with a good functional outcome [19]. Intramedullary ependymomas are tumors well-known to be treated surgically [14]. Grossly total resection of the tumor with adjuvant radiotherapy has been accepted as an optimal treatment for spinal ependymomas [14161718].
In this paper, we report a single case of cervicothoracic spinal ependymoma presenting with acute hemorrhage and resulting in acute paraplegia. There have been reports of ten patients with acute neurological deterioration caused by hemorrhagic spinal ependymoma (Table 1). However, our patient is the first case of a pregnant woman presenting with neurological symptoms immediately after delivery. In addition, a poorly demarcated heterogeneously enhancing lesion in her initial spinal MRI, as well as her history, made us hesitate about emergent surgery and to treat medically first. In our opinion, two hypotheses for this acute hemorrhage of spinal ependymoma could be considered. The first theory is that it could be caused by an acute decline of abdominal pressure due to the patient's cesarean delivery. As abdominal pressure falls down in an instant, hemodymanic change at the ependymoma could have caused internal bleeding. The second theory relates to the rapid change of intradural pressure. While no specific event during spinal epidural anesthesia was reported to occur during the patient's spinal epidural anesthesia, the epidural anesthesia was however not performed in our hospital. Therefore, we felt obliged to act with caution. If the needle had punctured the spinal dura during the procedure, and if the CSF had gushed out rapidly during the spinal epidural anesthesia, a rapid decline of intradural pressure could have caused internal bleeding within this spinal ependymoma.
As can be seen in Table 1, in most cases (in seven out of nine), the patients showed favorable outcomes. In addition, in most cases, an emergent surgery was performed. Only three patients underwent a delayed surgery. However, all patients who underwent delayed surgery showed excellent outcomes. All patients showed full neurological recovery. Concerning delayed surgery, Heuer et al. [8] noted that negative effect of the delayed surgery included extensive adhesions present at the resection. They noted that these adhesion increases the difficulty and length of the procedure. In our case, a severe adhesion of the tumor and the normal spinal cord tissue were observed as well. In addition, in our case, neurological symptoms of the patient were partially improving, although not as dynamically as in other reported cases of delayed surgery. Without any improvement of motor and bladder function, only sensory deficit showed improvement. The effect of early surgery on the final outcome remains unclear. However, in our patient, if we had been able to confirm the diagnosis of hemorrhagic spinal ependymoma by the initial spinal MRI, we could have performed emergent surgery and the neurological outcome could have been better.
To improve the neurologic symptoms of this patient, active physiotherapy and adjuvant radiotherapy could be considered as additional option [351114151617]. Compared with the patient after surgical treatment only, favorable outcome can be expected patient with surgery and dditional treatments.
In conclusion, this report shows the importance of recognizing acute hemorrhage of a spinal ependymoma as a possible cause of rapid neurological deterioration, even in young females immediately after a cesarean delivery under spinal anesthesia. Still, the effect of emergent surgery on the final outcome in these hemorrhagic spinal ependymomas remains unclear. However, where possible, emergent surgery should be recommended to reduce adhesion during surgery.
Figures and Tables
Fig. 1
Initial spinal magnetic resonance image (MRI) scan. A: T2-weighted MRI shows an abnormal spinal cord lesion from C2 level to D5. A spinal cord swelling with an irregular high signal intensity is noted in T2-weighted image. The margin is poorly demarcated. Most part of the lesion is suspicious of a syrinx formation or a hemorrhage. B: The lesion shows the low signal intensity in T1-weighted MRI. C: Gadolinium-enhanced T1-weighted MRI shows a heterogeneously enhancing lesion from C6 to D2. There is no abnormal intradural lesion in the proximal and distal areas.
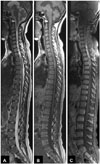
Fig. 2
Preoperative spinal MRI scan after 2 weeks of medical treatment. A: Preoperative gadolinium-enhanced T1-weighted MRI shows a heterogeneously enhancing mass from C3 level to D2 level. B: Axial cut of gadolinium-enhanced T1-weighted MRI shows a dark central portion with the margin enhancement.
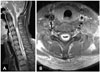
Fig. 3
Intraoperative findings. A: Unilateral hemilaminectomies were performed on the left side of C7 and T1 level. B: After opening the dura, a midline incision was done precisely at the spinal cord. A gray and sticky mass was observed below the midline pial incision (arrow). C: After removing the mass partially, a dark hematoma was observed inside the mass (arrow) and a large amount of the dark hematoma could be sucked out easily.
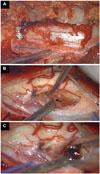
Fig. 4
Postoperative spinal MRI scan. A huge amount of the abnormal lesion decreased. A: T2-weighted MRI shows a hyper-intense lesion from C4 level to D2 level. B: T1-weighted MRI shows the same lesion in the low signal intensity. C: Enhancing portion is much more decreased in gadolinium-enhanced T1-weighted MRI as compared to the preoperative MRI scan. The remaining lesion is mainly from C7 to D2 level.
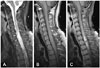
Fig. 5
Spinal MRI scan taken 1 week after the first surgery (preoperative MRI for the second surgery). A: Gadolinium-enhanced T1-weighted MRI shows that the enhancing mass diminished significantly and was partially removed at T1 level. B: Axial image of gadolinium-enhanced T1-weighted MRI shows a dark central portion which was the hematoma totally removed and the nhancing portion remained.
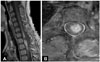
Fig. 6
Postoperative spinal MRI scan after the second surgery. A: Gadolinium-enhanced T1-weighted MRI shows that most of the enhancing portion was removed and only a very thin-layered enhancing portion remained. B: Axial image of gadolinium-enhanced T1-weighted MRI shows a thin-layered enhancing portion remaining at C7 level.
Table 1
Reported cases of hemorrhagic spinal ependymomas presenting with acute neurological deterioration

| Author (year) | Age/sex | Neurological symptoms | Predisposing factor | Location of lesion | Timing of surgery | Prognosis |
|---|---|---|---|---|---|---|
| Destée et al. (1984) [6] | 47/M | Cauda equina syndrome | Anticoagulant | L5–S1 | Delayed 1 week | Full recovery |
| Herb et al. (1990) [7] | 63/M | Cauda equina syndrome | N/A | L3 | Emergency | No improvement |
| Rivierez et al. (1990) [11] | 18/F | Cauda equina syndrome | N/A | L1–5 | N/A | N/A |
| Malbrain et al. (1994) [9] | 65/F | Cauda equina syndrome | Anticoagulant | L2–3 | Emergency | No improvement |
| Lagares et al. (2000) [13] | 24/M | Paraplegia | N/A | Low lumbar | Emergency | Improving |
| Oertel et al. (2000) [10] | 35/M | Paraplegia | N/A | D9–11 | Emergency | Improving |
| Tait et al. (2004) [12] | 57/F | Paraplegia | Anticoagulant | L3 | Emergency | Improving |
| Heuer et al. (2007) [8] | ||||||
| Case 1 | 31/F | Paraplegia | None | L1–S2 | Delayed 1 month | Full recovery |
| Case 2 | 31/M | Paraplegia | Heavy lifting | D11–L2 | Delayed 1 week | Full recovery |
| Martinez-Perez et al. (2012) [1] | 32/M | Paraplegia | None | D9 & L2–3 | Emergency | Improving |
| Present report (2016) | 24/F | Paraplegia | Delivery* | C2–D5 | Delayed 2 weeks | Sensory improving |
References
1. Martinez-Perez R, Hernandez-Lain A, Paredes I, Munarriz PM, Castaño-Leon AM, Lagares A. Acute neurological deterioration as a result of two synchronous hemorrhagic spinal ependymomas. Surg Neurol Int. 2012; 3:33.

2. Rawlings CE 3rd, Giangaspero F, Burger PC, Bullard DE. Ependymomas: a clinicopathologic study. Surg Neurol. 1988; 29:271–281.

3. Schweitzer JS, Batzdorf U. Ependymoma of the cauda equina region: diagnosis, treatment, and outcome in 15 patients. Neurosurgery. 1992; 30:202–207.
4. Yoshii S, Shimizu K, Ido K, Nakamura T. Ependymoma of the spinal cord and the cauda equina region. J Spinal Disord. 1999; 12:157–161.

5. Admiraal P, Hazenberg GJ, Algra PR, Kamphorst W, Wolbers JG. Spinal subarachnoid hemorrhage due to a filum terminale ependymoma. Clin Neurol Neurosurg. 1992; 94:69–72.

6. Destée A, Lesoin F, Warot M, Mendolia G, Devos P, Warot P. [Tumoral spinal meningeal hemorrhage during anticoagulant treatment]. Rev Neurol (Paris). 1984; 140:517–519.
7. Herb E, Schwachenwald R, Nowak G, Müller H, Reusche E. Acute bleeding into a filum terminale ependymoma. Neurosurg Rev. 1990; 13:243–245.

8. Heuer GG, Stiefel MF, Bailey RL, Schuster JM. Acute paraparesis from hemorrhagic spinal ependymoma: diagnostic dilemma and surgical management. Report of two cases and review of the literature. J Neurosurg Spine. 2007; 7:652–655.

9. Malbrain ML, Kamper AM, Lambrecht GL, et al. Filum terminale ependymoma revealed by acute cauda equina compression syndrome following intratumoral and spinal subarachnoid hemorrhage in a patient on oral anticoagulants. Acta Neurol Belg. 1994; 94:35–43.
10. Oertel J, Gaab MR, Piek J. Partial recovery of paraplegia due to spontaneous intramedullary ependyma haemorrhage. Acta Neurochir (Wien). 2000; 142:219–220.

11. Rivierez M, Oueslati S, Philippon J, et al. [Ependymoma of the intradural filum terminale in adults. 20 cases]. Neurochirurgie. 1990; 36:96–107.
12. Tait MJ, Chelvarajah R, Garvan N, Bavetta S. Spontaneous hemorrhage of a spinal ependymoma: a rare cause of acute cauda equina syndrome: a case report. Spine (Phila Pa 1976). 2004; 29:E502–E505.
13. Lagares A, Rivas JJ, Lobato RD, Ramos A, Alday R, Boto GR. Spinal cord ependymoma presenting with acute paraplegia due to tumoral bleeding. J Neurosurg Sci. 2000; 44:95–97. discussion 97-8.
14. Klekamp J. Spinal ependymomas. Part 1: Intramedullary ependymomas. Neurosurg Focus. 2015; 39:E6.

15. Kucia EJ, Bambakidis NC, Chang SW, Spetzler RF. Surgical technique and outcomes in the treatment of spinal cord ependymomas, part 1: intramedullary ependymomas. Neurosurgery. 2011; 68:1 Suppl Operative. 57–63. discussion 63.

16. Lee SH, Chung CK, Kim CH, et al. Long-term outcomes of surgical resection with or without adjuvant radiation therapy for treatment of spinal ependymoma: a retrospective multicenter study by the Korea Spinal Oncology Research Group. Neuro Oncol. 2013; 15:921–929.

17. Oh MC, Ivan ME, Sun MZ, et al. Adjuvant radiotherapy delays recurrence following subtotal resection of spinal cord ependymomas. Neuro Oncol. 2013; 15:208–215.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download