Abstract
We present the case of a 9-year-old boy with a non-germinomatous germ cell tumor (NGGCT) in the pineal gland that exhibited a fulminant course following chemo- and radiotherapy. After the detection of the tiny cerebellar enhancing nodule at the end of chemo- and radiotherapy, tumor seeding progressed rapidly into the entire cisternal space. We herein report a rare case of NGGCT with fulminant clinical course of concomitant cerebellar seeding, with review of literature.
Intracranial germ cell tumors (GCTs) are divided into germinomas and non-germinomatous GCTs (NGGCTs) according to the histologic components and the degree of differentiation [1]. NGGCTs constitute approximately one-third of intracranial GCTs, and exhibited worse prognoses than germinomas [123].
Multimodal approaches, including chemotherapy, radiotherapy, and surgical resection, have been attempted in many combinations to improve the dismal prognoses of intracranial NGGCTs [1456789]. Herein, we present the fulminant course of an intracranial NGGCT. Despite the standard chemotherapy and radiotherapy, a cerebellar nodule was detected, and the cerebrospinal fluid (CSF) seeding rapidly progressed into the whole cistern subsequently.
A 9-year-old boy presented diplopia for 15 days. In neurologic examination, he had an upward gaze limitation and sluggish light reflexes in both eyes. Magnetic resonance imaging (MRI) from an outside hospital revealed a 3 cm mass in the pineal gland. The mass exhibited a low signal intensity on T1-weighted imaging (T1WI) and a high signal intensity on T2-weighted imaging (T2WI). Contrast-enhanced T1WI revealed a strong enhancement of the lesion (Fig. 1). There was no evidence of leptomeningeal seeding in the spine on MRI. A tumor marker study revealed remarkably elevated level of alpha-fetoprotein (AFP) in both the serum (10,500 ng/mL) and CSF (8,300 ng/mL). However, the level of human chorionic gonadotropin in the serum was within the normal range.
Based on the MRI and tumor marker study [1], the pineal mass was diagnosed as a NGGCT. Therefore, the patient underwent chemotherapy and radiotherapy.
He underwent chemotherapy using the Korean Society of Pediatric Neuro-Oncology (KSPNO) protocol for high-risk GCTs (KSPNO-G052). He was treated with 2 cycles of alternating regimens (A and B). Regimen A was composed of carboplatin (450 mg/m2) on day 0, etoposide (150 mg/m2) on days 0, 1, and 2, and bleomycin (15 mg/m2) on day 2. Regimen B was composed of etoposide (150 mg/m2) on days 0, 1, and 2, cyclophosphamide (2,000 mg/m2) on days 0, and 1, and bleomycin (15 mg/m2) on day 2.
One month after chemotherapy, he underwent radiotherapy. The initial radiation was administered to the craniospinal axis at a dose of 30.6 Gy divided into 17 fractions over 3 weeks and subsequently to the pineal gland area at 23.4 Gy divided into 13 fractions over 3 weeks.
One month after the last radiotherapy, the serum AFP level decreased from 1,194 ng/mL to 187.2 ng/mL. MRI revealed a decreased in the size of mass to 1.38 cm. However, a new enhancing nodule appeared in the right cerebellar tonsil (Fig. 2B).
We were not convinced that the tiny nodule should be a leptomeningeal seeding because the tumor marker had decreased. The diplopia was resolved after the chemotherapy, and no other new symptoms appeared. Also, other CSF spaces were free from seeding. Due to the uncertain identity of the nodule, we planned a short-term follow up.
Two weeks after the detection of the cerebellar nodule, a tumor marker study revealed elevated AFP levels in both the serum and CSF. The serum AFP level was increased by four-fold (187.2 ng/mL to 879 ng/mL). The CSF AFP level was also markedly elevated (1,050 ng/mL). Therefore we planned a resection of the residual mass in the pineal gland. After examining the pathology of the pineal mass, additional radiotherapy was considered to treat the cerebellar nodule.
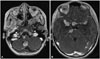
One month after the detection of the nodule, the patient was admitted for planned surgery. Unexpectedly, navigation MRI revealed an extensive CSF seeding including the prepontine, sylvian and suprasellar cisterns (Fig. 3). The surgery was cancelled.
He was scheduled for treatment according to the KSPNO protocol for relapsed/disseminated intracranial GCT (KSPNO-S-053). This protocol includes salvage chemotherapy with alternating A and B regimens for 4 cycles, tandem high-dose chemotherapy and autologous stem cell transplantation (HDCT/ASCT). Regimen A is composed of cisplatin (60 mg/m2) on day 0; etoposide (50 mg/m2) on days 0, 1, and 2; cyclophosphamide (750 mg/m2) on days 1 and 2; and vincristine (1.5 mg/m2) on days 0 and 7. Regimen B is composed of carboplatin (200 mg/m2) on days 0 and 1; etoposide (50 mg/m2) on days 0, 1, 2, 3, and 4; ifosfamide (750 mg/m2) on days 0, 1, 2, 3, and 4; and vincristine (1.5 mg/m2) on days 0 and 7 [10].
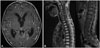
During the salvage chemotherapy, His clinical status deteriorated rapidly. After the 2nd cycle, a brain and spinal MRI revealed extensive CSF seeding across all of the cisternal spaces and the entire spinal canal (Fig. 4). Due to the extensive state of tumors, further chemotherapy was discontinued. The patient died three month after the salvage chemotherapy was initiated.
Previously, the 5-year overall survival rate for intracranial NGGCT has only been 20.40% with radiation therapy [211]. Unlike germinomas, NGGCTs respond poorly to radiation therapy. Therefore, the addition of chemotherapy to radiotherapy has been performed for several years [6]. Neoadjuvant chemotherapy with response-based radiotherapy has shown a three-year overall survival rate of 79% [12].
Furthermore, neoadjuvantly combined chemo- and radiotherapy followed by complete resection of the residual tumor has been reported to have a five-year overall survival rate of 93% [7]. Resection of the residual tumor after the initial treatment has been advocated by some groups [613]. Weiner et al. [1414] supported the surgical resection of residual masses after chemotherapy. Additionally, Kochi et al. [5] reported good survival with neoadjuvant chemo- and radiotherapy followed by resection of the residual tumor. They found viable tumor cells in three of nine residual tumors. Therefore, resection of the residual tumor seems to provide an oncological advantage for the elimination of the source of tumor progression. The present case exhibited a residual mass with elevated tumor marker levels following the chemo- and radiotherapy. In this condition, aggressive surgical treatment might be helpful for achieving better clinical outcomes.
In contrast to the treatment protocol for primary NGGCTs, there is no clear consensus regarding the management of relapsed and progressed cases [15]. Some groups have adopted HDCT/ASCT for the treatment of these tumors [1516]. Baek et al. [15] studied the effectiveness of HDCT/ASCT in eleven progressive or relapsed NGGCT cases. The 3-year overall survival rate was 36.4%. Additionally, Modak et al. [16] studied 12 cases, and the 4-year overall survival rate was 42%. In present study, HDCT/ASCT was planned. However, the tumor progression was too fulminant to initiate the HDCT/ASCT in time. If HDCT/ASCT had been considered earlier, the patient might have received HDCT/ASCT, and the outcome might have been better.
To alleviate fulminant progression of NGGCT after initial therapy, residual tumor should be observed closely and treated aggressively. The tracking of tumor marker levels could be a guide to clinical decision. It has been used as a sensitive indicator of recurrence and tumor viability [17]. In present case, leptomeningeal seeding should have been suspected at the time of initial detection of the cerebellar nodule, because the level of AFP had not normalized. Retrospectively, it seems that urgent application of active treatment such as surgical resection or HDCT/ASCT may have improved the outcome of the patient.
The treatment outcomes of NGGCTs are much worse than those of germinomas. Even when the initial therapy encompasses chemotherapy and radiotherapy, some NGGCTs can progress rapidly. To identify fulminant NGGCTs in advance, tumor marker studies and imaging to search for evidence of seeding are necessary. If the tumor marker is not normalized after the initial therapy and tumor seeding is suspected based on imaging, active management, including resective surgery or HDCT/ASCT, should be considered.
Figures and Tables
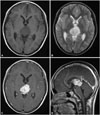 | Fig. 1Initial images. MR image showing the pineal mass, which is hypointense on T1- (A) and hyperintense on T2-weighted images (B). The mass is strongly enhanced following the injection of gadolinium (C: axial, D: sagittal image). |
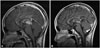 | Fig. 2Post-therapy images. Midsagittal T1-weighted MR image with gadolinium enhancement after chemotherapy (A) and radiotherapy (B). A tiny enhancing nodule (arrow) appears in the right paramedian cerebellar tonsil. |
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare of the Republic of Korea (grant number: HI12C0066).
References
1. Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. 2008; 13:690–699.

2. Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985; 63:155–167.

4. Kim JW, Kim WC, Cho JH, et al. A multimodal approach including craniospinal irradiation improves the treatment outcome of high-risk intracranial nongerminomatous germ cell tumors. Int J Radiat Oncol Biol Phys. 2012; 84:625–631.

5. Kochi M, Itoyama Y, Shiraishi S, Kitamura I, Marubayashi T, Ushio Y. Successful treatment of intracranial nongerminomatous malignant germ cell tumors by administering neoadjuvant chemotherapy and radiotherapy before excision of residual tumors. J Neurosurg. 2003; 99:106–114.

6. Millard NE, Dunkel IJ. Advances in the management of central nervous system germ cell tumors. Curr Oncol Rep. 2014; 16:393.

7. Nakamura H, Makino K, Kochi M, Ushio Y, Kuratsu J. Evaluation of neoadjuvant therapy in patients with nongerminomatous malignant germ cell tumors. J Neurosurg Pediatr. 2011; 7:431–438.

8. Ogawa K, Toita T, Nakamura K, et al. Treatment and prognosis of patients with intracranial nongerminomatous malignant germ cell tumors: a multiinstitutional retrospective analysis of 41 patients. Cancer. 2003; 98:369–376.

9. Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol. 1997; 32:71–80.
10. Park JE, Kang J, Yoo KH, et al. Efficacy of high-dose chemotherapy and autologous stem cell transplantation in patients with relapsed medulloblastoma: a report on the Korean Society for Pediatric Neuro-Oncology (KSPNO)-S-053 study. J Korean Med Sci. 2010; 25:1160–1166.

11. Hoffman HJ, Otsubo H, Hendrick EB, et al. Intracranial germ-cell tumors in children. J Neurosurg. 1991; 74:545–551.

12. Kretschmar C, Kleinberg L, Greenberg M, Burger P, Holmes E, Wharam M. Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2007; 48:285–291.

13. Souweidane MM, Krieger MD, Weiner HL, Finlay JL. Surgical management of primary central nervous system germ cell tumors: proceedings from the Second International Symposium on Central Nervous System Germ Cell Tumors. J Neurosurg Pediatr. 2010; 6:125–130.
14. Weiner HL, Lichtenbaum RA, Wisoff JH, et al. Delayed surgical resection of central nervous system germ cell tumors. Neurosurgery. 2002; 50:727–733. discussion 733-4.

15. Baek HJ, Park HJ, Sung KW, et al. Myeloablative chemotherapy and autologous stem cell transplantation in patients with relapsed or progressed central nervous system germ cell tumors: results of Korean Society of Pediatric Neuro-Oncology (KSPNO) S-053 study. J Neurooncol. 2013; 114:329–338.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download