Abstract
Cellular neurothekeoma (CNT) is an uncommon variant of neurothekeoma that is composed of pithelioid to spindled cells with variable nuclear atypia or pleomorphism but no myxoid stroma. CNT occurs predominantly in the head and neck or upper trunk of children and young adults, with female predominance. The following case is different from typical CNTs. An 88-year-old female presented with a palpable mass on the scalp, which we excised. Histologically, the tumor was non-encapsulated and composed of spindled and epithelioid cells arranged in fascicles and nodules separated by a collagen-rich stroma. Immunohistochemical analysis showed that the epithelioid and spindle-shaped cells were focally positive for vimentin, neuron-specific enolase, smooth muscle actin, CD68, and CD10 but negative for S-100 protein, HMB-45, epithelial membrane antigen, and CD34. We report a new case of CNT that arose in the scalp of an older patient and that was associated with uncommon clinical, histological, and immunohistochemical profiles.
Neurothekeoma (NT) is a benign skin tumor that is usually related to a myxoid neoplasm of the soft tissue with presumed neural differentiation [123]. NTs usually occur in the first or second decade of life and have a female dominance [1]. Approximately 30% of these lesions involve the head and neck; however, they may less frequently involve extracutaneous sites, such as breast, oral cavity, spinal intradural space, and hypopharynx [145678]. There are two histopathologic variants: classic (myxoid) and cellular, although cellular neurothekeoma (CNT) was recently determined to be histogenetically unrelated to classic NT [29].
We report a new case of CNT that arose in the scalp of an older patient and that was associated with uncommon clinical, histological, and immunohistochemical profiles.
An 88-year-old woman presented to our hospital with a painless, palpable mass in the left parietal area of the scalp that had shown a slow increase in size to 6 cm in diameter over two years. Radiologic evaluations with computed tomography and magnetic resonance imaging demonstrated a well-defined, heterogeneous, enhancing mass in the left parietal convexity with focal cortical defect of the left parietal bone (Fig. 1A).
Surgical excision of the lesion was performed under general anesthesia. A hard, whitish-grey mass with little bleeding was completely removed (Fig. 1B). The scattered calcified lesions of the tumor bed were drilled out.
Histopathologically, the tumor was non-encapsulated and composed of epithelioid and spindle-shaped cells separated by prominent fibrous septa were observed in fascicles or nodules with a geographical pattern (Fig. 2A). Also, this tumor was composed of nests and bundles of epithelioid to spindle cells with sparse myxoid matrix (Fig. 2B). The tumor cells had oval or round vesicular nuclei and abundant pale eosinophilic cytoplasm with ill-defined cell borders.
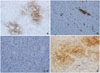
Some cells had nuclear pleomorphism with prominent nuclei, mild to moderate cellular pleomorphism including giant cells is seen (Fig. 2C). Mitotic activity is low (3/10 high power field) (Fig. 2D). Distinct perineural invasion and tumor necrosis are not presented. The tumor involved the margins of the excision and infiltrated into fat and skeletal muscle. Immunohistochemical analysis showed that the epithelioid and spindle-shaped cells were at least focally positive for vimentin, neuron-specific enolase (NSE) (Fig. 3A), smooth muscle actin (SMA) (Fig. 3B), CD68 (Fig. 3C), and CD10 (Fig. 3D) but negative for S-100 protein, HMB-45, epithelial membrane antigen (EMA), and CD34. On the basis of these findings, a diagnosis of CNT was made.
NT is an unusual benign cutaneous tumor involving the dermis and is composed of distinct lobules of bland spindle cells separated by fibrous connective tissue [1011]. CNT is an uncommon variant of NT that is hypercellular and lacks myxoid stroma [2]. Typically, CNTs occur as a solitary, asymptomatic papule during the first three decades of life, demonstrating a female predominance. The tumors most often arise on the upper extremities and head and neck [91213]. The mean tumor size is 1 cm, and 90% are smaller than 2 cm [9]. CNTs are generally poorly marginated, micronodular, or lobulated neoplasms composed of epithelioid to spindled cells with abundant pale eosinophilic cytoplasm. NTs and CNTs are similarly immunoreactive for vimentin, NKI/C3, CD10, SMA, and CD68 and are negative for HMB-45, cytokeratin, desmin, Melan A, and S-100 protein [210]. In our case, such a tumor was found on the scalp of an elderly woman and was very large at 6 cm in size. These characteristics were different from any other previously reported CNTs.
CNTs might be mistaken for malignant tumors, such as sarcomas, because of their hypercellularity, the presence of nuclear atypia, and the extension into fat or skeletal muscle [2]. The chief differential diagnostic considerations for CNTs are dermal nerve sheath myxoma (DNSM), also called myxoid NT. DNSM is a lobulated tumor composed of variably epithelioid to spindled or stellate cells in an abundant myxoid matrix, whereas CNTs can show focally myxoid stroma or rare extensive myxoid change [9]. While CNTs show irregular, infiltrative margins within surrounding dermal collagen or subcutaneous adipose tissue, the lobules of DNSM are very sharply demarcated. In pathologic aspect, other common differential diagnoses for CNTs include classic (myxoid) NT, fibrohistiocytic tumors, melanocytic tumors, cellular perineuroma. Unlike this case, myxoid NT shows prominent myxoid stroma (>50% myxoid matrix) and expression of S-100 [9121314]. The most helpful pathologic finding in support of CNTs, comparing with fibrohistiocytic tumors, is nested patterns of epitheliod and more plump spindle tumor cells with vesicular or open-chromated nuclei and indistinct cell borders. In addition, the positive reactions for NSE and CD10 and lack of expression of CD34 are helpful to differential diagnosis. Melanocytic tumors show almost always positive reaction for HMB-45 and S-100, in contrast to this case. Cellular perienuroma expresses diffusely or focally EMA differentiated from this case. Based on the report of Fetsch et al. [10] showing a high recurrence rate of DNSM, this differential diagnosis is clinically important because CNTs rarely recur. The strong positive staining for neuroendocrine markers such as NSE, chromogranin, synaptophysin, and CD56 suggest possible neuroendocrine differentiation of the tumor cells [2]; however, Hornick and Fletcher [9] suggested that all tumors are diffusely positive for NKI/C3, and most (around 90%) are positive for NSE because these markers lack specificity. In our case, the tumor likely did not represent neuroendocrine differentiation because of its focally positive NSE.
CNTs are benign tumors with nuclear atypia and only occasionally recur locally when the tumor has been marginally excised or has involved excision margins. Incomplete excision of CNT can lead to recurrence; therefore, total excision is recommended. Large tumor size (>2 cm) and atypical histologic features including high mitotic rate, pleomorphism, and infiltration of fat seem to have no clinical significance [9].
In conclusion, CNTs are benign lesions that do not recur following total excision. Definitive diagnosis of CNT can be made with immunohistochemical analysis. Complete excision of the tumor with negative margins is curative.
Figures and Tables
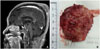 | Fig. 1A: T1-weighted, post-contrast, sagittal magnetic resonance imaging demonstrates a well-defined, heterogeneous, enhancing mass in the left parietal convexity with a focal cortical defect of the left parietal bone. B: A hard, whitish-grey mass with little bleeding was completely removed under general anesthesia. |
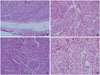 | Fig. 2Histopathologic examination revealed a non-encapsulated tumor (A: H&E, ×40) composed of nests and bundles of epithelioid to spindle cells with sparse myxoid matrix (B: H&E, ×100), arranged in fascicles or nodules, often in a geographic pattern, separated by a collagen-rich stroma (C: H&E, ×100). Mild to moderate pleomorphism of epithelioid cells with indistinct border including a giant cell and a mitotic figure is observed (D: H&E, ×200). H&E, hematoxylin and eosin. |
References
1. Cohen NA, Samadi DS, Pawel BR, Kazahaya K. Cellular neurothekeoma of the maxilla. Ann Otol Rhinol Laryngol. 2004; 113:384–387.

2. D'Antonio A, Cuomo R, Angrisani B, Addesso M, Angrisani P. Cellular neurothekeoma with neuroendocrine differentiation. Dermatol Online J. 2011; 17:2.
3. Pulitzer DR, Reed RJ. Nerve-sheath myxoma (perineurial myxoma). Am J Dermatopathol. 1985; 7:409–421.

4. Chow LT, Ma TK, Chow WH. Cellular neurothekeoma of the hypopharynx. Histopathology. 1997; 30:192–194.

5. Katsourakis M, Kapranos N, Papanicolaou SI, Patrikiou A. Nerve-sheath myxoma (neurothekeoma) of the oral cavity: a case report and review of the literature. J Oral Maxillofac Surg. 1996; 54:904–906.

6. Paulus W, Jellinger K, Perneczky G. Intraspinal neurothekeoma (nerve sheath myxoma). A report of two cases. Am J Clin Pathol. 1991; 95:511–516.

7. Vij M, Jaiswal S, Agrawal V, Jaiswal A, Behari S. Nerve sheath myxoma (neurothekeoma) of cerebellopontine angle: case report of a rare tumor with brief review of literature. Turk Neurosurg. 2013; 23:113–116.

8. Wee A, Tan CE, Raju GC. Nerve sheath myxoma of the breast. A light and electron microscopic, histochemical and immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1989; 416:163–167.
9. Hornick JL, Fletcher CD. Cellular neurothekeoma: detailed characterization in a series of 133 cases. Am J Surg Pathol. 2007; 31:329–340.

10. Fetsch JF, Laskin WB, Hallman JR, Lupton GP, Miettinen M. Neurothekeoma: an analysis of 178 tumors with detailed immunohistochemical data and long-term patient follow-up information. Am J Surg Pathol. 2007; 31:1103–1114.

11. Gallager RL, Helwig EB. Neurothekeoma--a benign cutaneous tumor of neural origin. Am J Clin Pathol. 1980; 74:759–764.

12. Lane JE, Mullins S, Davis LS. Clinical pathologic challenge. Cellular neurothekeoma. Am J Dermatopathol. 2006; 28:65–66. answer and discussion 87-8.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download