Abstract
Primary central nervous system lymphoma (PCNSL) is an extranodal Non-Hodgkin's lymphoma that is confined to the brain, eyes, and/or leptomeninges without evidence of a systemic primary tumor. Although the tumor can affect all age groups, it is rare in childhood; thus, its incidence and prognosis in children have not been well defined and the best treatment strategy remains unclear. A nine-year old presented at our department with complaints of diplopia, dizziness, dysarthria, and right side hemiparesis. Magnetic resonance image suggested a diffuse brain stem glioma with infiltration into the right cerebellar peduncle. The patient was surgically treated by craniotomy and frameless stereotactic-guided biopsy, and unexpectedly, the histopathology of the mass was consistent with diffuse large B cell lymphoma, and immunohistochemical staining revealed positivity for CD20 and CD79a. Accordingly, we performed a staging work-up for systemic lymphoma, but no evidence of lymphoma elsewhere in the body was obtained. In addition, she had a negative serologic finding for human immunodeficient virus, which confirmed the histopathological diagnosis of PCNSL. She was treated by radiosurgery at 12 Gy and subsequent adjuvant combination chemotherapy based on high dose methotrexate. Unfortunately, 10 months after the tissue-based diagnosis, she succumbed due to an acute hydrocephalic crisis.
Primary central nervous system lymphoma (PCNSL) is an extranodal Non-Hodgkin's lymphoma confined to the brain, eyes, and/or leptomeninges with no evidence of a primary tumor elsewhere in the body [1]. PCNSL can affect all age groups, and those without acquired immunodeficiency syndrome (AIDS) have a median age in the sixth decade [2]. This tumor is rare in childhood, and thus, the incidence and prognosis of PCNSL is not well defined in children, and the best treatment strategy remains unclear. In fact, among 596 cases of PCNSL reported to the Japanese Brain Tumor Registry between 1969 and 1990, only nine (1.5%) were in children [3]. In the US, 1% of all PCNSL were found to occur in patients younger than 19 years of age in the Surveillance, Epidemiology, and End Results program, which ran from 1973 to 1998 [4], giving an estimated incidence of 15 to 20 cases per year in North America. It is estimated that 14 cases of pediatric PCNSL will be reported annually to the newly opened Rare non-Hodgkin's lymphoma registry of Children's Oncology [5]. PCNSL tends to occur more frequently in immunodeficient children, and has an incidence of 0.57 to 1% in human immunodeficiency virus (HIV)-infected children [6] and of 4% in patients with a congenital immunodeficiency [7].
PCNSL is usually located in the craniospinal axis, particularly in the posterior fossa [1]. The most frequent locations are the frontal lobes, basal ganglia, and corpus callosum, and multiple lesions are seen in 20–40% of cases at presentation [89]. PCNSL lesions may be divided into parenchymal, subependymal, and leptomeningeal [10], and all present the possibility of ventricular involvement (2–8.6%) [1112], which nearly always occurs secondary to an extension from a subependymal location (the reported incidence of subependymal location is as high as 40–100% in cases with ventricular involvement) [1011]. The frontal lobe is the region of the brain most commonly involved and a brain stem location is extremely rare [13]. We were unable to find any report of PCNSL involving the brain stem, especially in childhood. Here, we report a case of PCNSL of the brain stem in an immunocom-petent child, and provide a review of literature.
A 9-year-old Korean girl was admitted to our hospital with a complaint of slowly progressive diplopia, dysarthria, gait disturbance, and weakness on the right side of her body which had worsened significantly over the previous 2 weeks. A physical examination revealed an obese girl with a body weight of 58 kg, who was not in acute distress, but was somewhat lethargic. Cardiovascular, respiratory, gastrointestinal, hematologic, skin, endocrine, and immunologic reviews were all negative. She had no history of congenital immunodeficiency disease, previous organ transplantation, or immunosuppressive therapy, and did not have AIDS. Her family history was unremarkable for cancer or any immunodeficiency. A neurological examination conducted at admission revealed intact visual acuity with a normal light reflex on both sides, oculomotor and abducent cranial nerve palsy on the right side, and left-sided motor paresis of grade 4/5 in both upper and lower extremities. The other cranial nerves were functionally intact, and the sensory system examination was essentially normal. Her Karnofsky performance scale (KPS) score was 70, and she had no specific medical history or developmental disorder.
A contrast-enhanced magnetic resonance image (MRI) scan of the brain demonstrated an irregular space-occupying lesion in the pons and cerebellar peduncle that enhanced moderately and heterogeneously with gadolinium (Gd) (Fig. 1). The scan also revealed that despite the location of the tumor, the cerebrospinal fluid (CSF) outflow pathway was not mechanically obstructed. Positron emission tomography-computed tomography (PET-CT) presented a hot uptake focus in the area of Gd enhancement in the previous MRI (Fig. 2). In view of the tumor's location, its MRI features, and the patient's age, craniotomy and frameless stereotactic-guided excisional biopsy of the enhancing lesion located in the right-sided cerebellar peduncle was performed using a neuronavigation system (Medtronic Navigation, Inc., Louisville, CO, USA) under a presumptive diagnosis of diffuse infiltrative brain stem glioma.
Unexpectedly, the histopathology of the lesion revealed B-cell malignant lymphoma. A section of biopsy tissue revealed that brain tissue had been diffusely infiltrated by large pleomorphic lymphoid cells (Fig. 3A). Rare mitotic figures were also seen. Tumor cells exhibited strong staining for CD20 (Fig. 3B) and CD79a (Fig. 3C), bur were negative for myeloperoxidase, glial fibrillary acidic protein, CD3, CD45R0, and CD68. Her MIB1 index (a surrogate of the number of actively proliferating cells) was 40%. Accordingly, a diagnosis of high grade, diffuse large cell non-Hodgkin's lymphoma of the B cell phenotype was established.
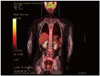
After confirming the histopathological diagnosis, we performed a systemic work-up for non-Hodgkin's lymphoma. However, whole craniospinal MRI and a CSF study did not reveal any evidence of additional neoplastic disease. A bone marrow biopsy, conducted by aspiration through the iliac crest, abdominal and chest CT scans, a bone scan, an ophthalmologic examination, including a slit lamp examination, and a whole body PET scan (Fig. 4) failed to demonstrate neoplasia elsewhere. Immunoglobulin levels were normal and ELISA for Epstein-Barr virus and acquired HIV were negative. Her laclactate dehydrogenase (LDH) level was below 450 mL. Therefore, the brain stem lesion was confirmed as a solitary manifestation of non-Hodgkin's lymphoma.
Postoperatively, the patient did well and achieved a relatively stable neurological state. Three weeks after biopsy, she received stereotactic radiosurgery with the Leksell gamma knife (Elekta Instruments, Atlanta, GA, USA). A dose of 12 Gy was delivered to the margin enclosed in the 50% isodense line. Great care was taken to avoid delivering more than 10 Gy to any part of the pons. At one month after radiosurgery, systemic chemotherapy was started using a high dose methotrexate (3.5 g/m2) based combination regimen with vincristine and procarbazine because of diffusely infiltrative tumor pattern. Although she completed 5 cycles of chemotherapy successfully, grade 3 leukocytopenia and thrombocytopenia as defined by the Common Terminology Criteria for Adverse Events occurred after the third cycle, and thus, the methotrexate dose was reduced by 25% during the fourth and fifth cycles. She tolerated the systemic chemotherapy and her neurologic state was stable throughout.
At her 3-month follow-up after chemotherapy, her KPS had improved to 90 from 70 at presentation, and she no longer complained of diplopia. She was also able to walk independently without the aid of cane and was more alert. An MRI scan revealed that the enhancing lesion in the brain stem and cerebellar peduncle had disappeared (Fig. 5), which concurred with her improved clinical condition and neurological status. However, at 5 months after chemotherapy, she was referred to the emergency room at our hospital due to acute altered consciousness. She was comatose and her pupils were fully dilated. A CT scan of the head revealed an acute hydrocephalus crisis, which was treated by extraventricular drainage. However, she did not recover from her moribund neurological state and died several weeks after admission. Her survival duration was 10 months.
PCNSL is a very rare brain tumor in children, and it has been estimated that only 14 cases occur annually in the United States [5]. In Korea, no case of PCNSL has been reported in a child of less than 10 years, although several reports have been issued on PCNSL in adulthood. In terms of its location, we found only one case of PCNSL located in the brain stem in the Korean literature [14], in this case, although the exact age was not provided, the patient was at least 17 years old. To identify prior cases of PCNSL with a brain stem location in children, we searched the PubMed database using the key words "primary, central nervous system, lymphoma, childhood, and brain stem". However, we were unable to locate a single article.
Because of its rarity, the optimal treatment of PCNSL in children has not established and much of the available information is derived from small pediatric case series or has been extrapolated from larger adult series. Although several trials have been conducted on the treatment of pediatric PCNSL by radiotherapy alone, cure rates have been low and local recurrence rates high. Median reported overall survivals range from 11.6 to 15.2 months [151617]. Although we did not find any reports on children regarding the role of radiosurgery, it has come to the fore as a therapeutic modality for the treatment of PCNSL with brain stem involvement. Campbell et al. [18] reported a case of PCNSL in the brain stem that was managed by gamma knife radiosurgery. In this case, a dose of 11 Gy was delivered to the tumor margin and follow-up MRI at 2 months postoperatively revealed a 50% reduction in the size of the enhancing lesion. Han et al. [19] presented their preliminary results for Novalis radiosurgery for PCNSL in elderly patients. They concluded that this radiosurgery probably provides a safe and effective therapeutic alternative treatment for PCNSL in the elderly. In presented case, we used gamma knife radiosurgery at 12 Gy. The patient well tolerated this treatment neurologically, and developed no radiosurgery-associated complications.
The effectivenesses of combination chemotherapy alone, before, or after cerebral irradiation have been studied [2021] and the introduction of methotrexate-based regimens and cranial radiotherapy has been reported to improve the prognosis of immunocompetent patients with PCNSL [2223]. Abla et al. [5] described ten cases of pediatric PCNSL treated with chemotherapy alone to prevent cranial radiation-induced cognitive dysfunction and secondary malignant neoplasm development. Most of their patients received high-dose methotrexate and cytarabine, and they achieved a 5-year event-free survival rate of 70%. Nevertheless, despite these advances, the best therapeutic strategy for children with PCNSL remains unclear.
According to the International Extranodal Lymphoma Study Group for PCNSL, an age of more than 60 years, a performance status (PS) greater than 1, an elevated LDH serum level, a high CSF protein concentration, and involvement of deep brain regions (periventricular regions, basal ganglia, brainstem, and/or cerebellum) are significantly and independently associated with poorer survival [24]. In this previous study, these five variables were used to design a prognostic scoring system. Each variable was assigned a value of either 0, if favorable, or 1, if unfavorable, and values were then added to arrive at final sc-ores. Patients with final scores of 0 or 1 were defined as low risk, those with scores of 2 or 3 as intermediate risk, and those with scores of 4 or 5 as high risk. For our patient, age at presentation was 9 years, her PS was 1, which meant restricted strenuous physical activity but able to ambulate and carry out work of a light or sedentary nature, her serum LDH level was 180 mL, and her CSF protein concentration was normal. Thus, her only poor prognostic factor was a brain stem location, and her final score was 1, indicating low risk. However, although she underwent successful radiotherapy and adjuvant chemotherapy, her the survival duration was only 10 months. We cannot explain why the acute hydrocephalic crisis occurred.
We report a rare case of PCNSL of the brain stem in an immunocompetent child. Despite aggressive radiotherapy and adjuvant chemotherapy, PCNSL of the brain stem is difficult to be treated. This tumor is very rare in childhood, and thus, its incidence and prognosis in children are not well defined and the best treatment strategy remains unclear. A better understanding of the characteristics and features of this brain stem lesion is required, and an optimal therapeutic strategy is needed to improve survival.
Figures and Tables
Fig. 1
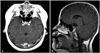
Preoperative axial (A) and sagittal (B) gadolinium-enhanced T1-weighted magnetic resonance image showing a poorly demarcated heterogeneous enhanced mass in the pons and cerebellar peduncle. There was no mechanical obstruction of the cerebrospinal fluid outflow pathway.
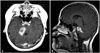
Fig. 2
Positron emission tomography-computed tomography of brain presented two hot uptake foci in the area of gadolinium-enhancement in the previous magnetic resonance image, that is, in the pons and cerebellar peduncle.
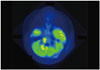
Fig. 3
Histopathological microphotographs of non-Hodgkin's lymphoma. Section of biopsy tissue revealing diffuse infiltration of brain parenchyma by large pleomorphic lymphoid cells (A) (hematoxylin and eosin stained; original magnification ×40). Rare mitotic figures were also seen. Tumor cells exhibited strong staining for CD20 (B) and CD79a (C).

References
2. Herrlinger U, Schabet M, Bitzer M, Petersen D, Krauseneck P. Primary central nervous system lymphoma: from clinical presentation to diagnosis. J Neurooncol. 1999; 43:219–226.
3. Kai Y, Kuratsu J, Ushio Y. Primary malignant lymphoma of the brain in childhood. Neurol Med Chir (Tokyo). 1998; 38:232–237.
4. Kadan-Lottick NS, Skluzacek MC, Gurney JG. Decreasing incidence rates of primary central nervous system lymphoma. Cancer. 2002; 95:193–202.

5. Abla O, Sandlund JT, Sung L, et al. A case series of pediatric primary central nervous system lymphoma: favorable outcome without cranial irradiation. Pediatr Blood Cancer. 2006; 47:880–885.

6. Rodriguez MM, Delgado PI, Petito CK. Epstein-Barr virus-associated primary central nervous system lymphoma in a child with the acquired immunodeficiency syndrome. A case report and review of the literature. Arch Pathol Lab Med. 1997; 121:1287–1291.
7. Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999; 43:199–201.
8. Go JL, Lee SC, Kim PE. Imaging of primary central nervous system lymphoma. Neurosurg Focus. 2006; 21:E4.

9. Hochberg FH, Baehring JM, Hochberg EP. Primary CNS lymphoma. Nat Clin Pract Neurol. 2007; 3:24–35.

10. Park SW, Yoon SH, Cho KG. An endoscopically proven ventriculitis-type, cyst-like intraventricular primary lymphoma of the central nervous system. Acta Neurochir (Wien). 2006; 148:981–984.

11. Bühring U, Herrlinger U, Krings T, Thiex R, Weller M, Küker W. MRI features of primary central nervous system lymphomas at presentation. Neurology. 2001; 57:393–396.

12. Coulon A, Lafitte F, Hoang-Xuan K, et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol. 2002; 12:329–340.

13. Erdag N, Bhorade RM, Alberico RA, Yousuf N, Patel MR. Primary lymphoma of the central nervous system: typical and atypical CT and MR imaging appearances. AJR Am J Roentgenol. 2001; 176:1319–1326.
14. Kwon HD, Huh R, Kim DS, Park YG, Choi JU, Chung SS. Primary central nervous system lymphoma: clinical analysis and prognostic factors. J Korean Neurosurg Soc. 2000; 29:1628–1633.
15. Henry JM, Heffner RR Jr, Dillard SH, Earle KM, Davis RL. Primary malignant lymphomas of the central nervous system. Cancer. 1974; 34:1293–1302.

16. Ishikawa H, Hasegawa M, Tamaki Y, et al. Comparable outcomes of radiation therapy without high-dose methotrexate for patients with primary central nervous system lymphoma. Jpn J Clin Oncol. 2003; 33:443–449.

17. Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992; 23:9–17.

18. Campbell PG, Jawahar A, Fowler MR, Delaune A, Nanda A. Primary central nervous system lymphoma of the brain stem responding favorably to radiosurgery: a case report and literature review. Surg Neurol. 2005; 64:400–405. discussion 405.

19. Han SR, Yee GT, Choi CY, Sohn MJ, Lee DJ, Whang CJ. Novalis radiosurgery of primary central nervous system lymphoma in elderly patients : preliminary results. J Korean Neurosurg Soc. 2006; 39:409–412.
20. Brada M, Dearnaley D, Horwich A, Bloom HJ. Management of primary cerebral lymphoma with initial chemotherapy: preliminary results and comparison with patients treated with radiotherapy alone. Int J Radiat Oncol Biol Phys. 1990; 18:787–792.

21. DeAngelis LM, Yahalom J, Thaler HT, Kher U. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992; 10:635–643.

22. Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006; 24:5711–5715.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download