Abstract
When treating childhood acute lymphoblastic leukemia (ALL), secondary neoplasms are a significant long term problem. Radiation is generally accepted to be a major cause of the development of secondary neoplasms. Following treatment for ALL, a variety of secondary tumors, including brain tumors, hematologic malignancies, sarcomas, thyroid cancers, and skin cancers have been reported. However, oligodendroglioma as a secondary neoplasm is extremely rare. Herein we present a case of secondary oligodendroglioma occurring 13 years after the end of ALL treatment.
One of the serious late consequences of successful childhood acute lymphoblastic leukemia (ALL) treatment is the development of a secondary neoplasm, which is defined as another malignant tumor arising in close proximity to, or remote from, the primary tumor at a different point in time and with an independent oncogenesis. Whether, in individual cases, the development of a secondary neoplasm is related to the treatment of the previous one is uncertain, because genetic risk factors or other external carcinogens may also be involved. However, it is certain that the risk of a secondary neoplasm developing in patients treated for ALL is higher than that of the normal population and, in fact, a variety of secondary neoplasms, such as brain tumors, hematologic malignancies, sarcomas, thyroid cancers, and skin cancers have been reported in the literature [7131423]. Radiation effects are generally accepted to be a crucial factor in the development of the secondary neoplasm, but the exact mechanism by which this occurs remains unclear [29].
Here, we present an unusual case of low-grade oligoden-droglioma [World Health Organization (WHO II)] occurring in a patient 13 years after undergoing chemotherapy and prophylactic cranial irradiation for ALL.
A 21-year-old male was admitted to our institution due to a 1-month history of a newly started simple partial seizure. He had a history of successful recovery from ALL with systemic chemotherapy (vincristine, L-asparaginase, 6-mercaptopurine, calcort, doxorubicin), intrathecal methotrexate, and cranial irradiation (18 Gy delivered in ten fractions over a period of 12 days). Calcort was not used in the maintenance therapy. A mass with 2 cm in the largest diameter was seen in the right insular cortex on the contrast-enhanced brain MRI with no post-contrast enhancement (Fig. 1). A navigation-guided biopsy obtained sufficient tumor tissue and the postoperative course was uneventful.
The histological study revealed a grade II oligodendroglioma according to the WHO classification [20]: the ×400 magnified pathological examination observed round uniform nuclei, nucleoli, and perinuclear halos (Fig. 2). Further molecular analysis revealed the loss of heterozygosity (LOH) of chromosomes 1p and 19q (Fig. 3), and positive for the isocitrate dehydrogenase 1 mutation at residue 132:pR132H (tumor proportion of the tested tissue: 70%) (Fig. 4).
Due to the previous irradiation history, additional radiation therapy was not suitable for the patient.
Thus, the patient was treated with a chemotherapeutic regimen consisting of procarbazine, lomustine, and vincristine, rather than surgery or re-radiation due to the fact that the patient had a tumor with 1p19q loss. His general condition was well maintained throughout the course of the treatment, and he was able to complete the 6th chemotherapy cycle as initially planned. The patient had been successfully treated for ALL 13 years earlier. The patient was treated with anticonvulsants for a year after the diagnosis of the oligodendroglioma. The patient has been doing well and his tumor as shown by imaging at his last follow up, 3 years after the diagnosis, is under control (Fig. 5).
Intensive multidrug therapy with prophylactic cranial irradiation has steadily improved the overall survival of children with ALL. However, long-term survivors of childhood ALL are also at risk for the development of a secondary neoplasm [7131423]. A recent investigation in Japan has found secondary neoplasms development in 37 (2.2%) of 1,716 patients treated after a diagnosis of ALL within a median follow-up duration of 9.5 years [14]. The types of secondary neoplasms in these patients includes hematologic malignancies (acute myeloid leukemia, myelodysplastic syndrome and non-Hodgkin lymphoma), brain tumors and other solid carcinomas. This risk is likely to be the consequence of multiple factors, including environmental factors and genetic susceptibility related to the primary diagnosis, radiotherapy, family history of cancer, immunosuppression, and hormonal factors [1236].
Multiple previous cohort studies have proposed a possible correlation between cranial radiation and the development of secondary neoplasms in patients with ALL. Among 9,720 patients who received treatment according to the protocols of the Children’s Cancer Study Group, 24 patients developed secondary neoplasms; 18 gliomas, 2 meningiomas, and 4 primitive neuroectodermal tumors (PNET). The relative risk of developing secondary neoplasms in patients with an irradiation history is 22-fold higher when compared to the normal population, and demonstrated an inverse relationship between the incidence of the tumor and the age of radiation exposure. This was corroborated by the finding of a 19-fold relative risk of a secondary neoplasm in a German cohort of 5,006 patients, in which 8 gliomas, 2 meningiomas, and 3 PNETs had occurred [19]. According to a cohort study involving 361 patients at Saint Jude Children’s Research Hospital, none of whom received cranial irradiation therapy, none of the patients developed a secondary neoplasm following treatment. In contrast, a number of studies, including the aforementioned, have reported a significantly increased risk of developing a secondary neoplasm with increasing doses of radiation treatment doses [2834].
By reviewing the literature, we found 24 published cases of low-grade gliomas that could be related to previous irradiation treatments for various pathologies (Table 1), but of those only a single case was a low-grade oligodendroglioma [34]. A 2 year-old male underwent a combination of chemotherapy and radiation therapy and received a total of 24 Gy of radiation to the cranial region after being diagnosed with central nervous system (CNS) leukemia. After a nine-year latency period, he was diagnosed with a low-grade oligodendroglioma [34].
Generally, the following diagnostic criteria are used for radiation-induced tumors: new lesion arises within the field of radiation and it is histologically different from the primary tumor; the new lesion arises during the latency period after the treatment of the primary tumor, which permits the secondary tumor to develop. Also, patients have no other predisposing risk factors besides the history of radiotherapy [27]. Our patient satisfied all of the criteria; the oligodendroglioma occurred in the frontal lobe during the latency period in the region previously exposed to radiation. In addition, besides the radiotherapy in his childhood, the patient had no other risk factors for brain tumors such as genetic or environmental factors. According to previous studies, the average latency period of secondary high-grade gliomas is known to be 9.1 years to 11 years, whereas it takes 19 years in average for meningiomas to develop [162434].
Although untrue for the patient of the present case, it must be noted that a history of ALL during childhood represents a possible risk factor for the development of secondary neoplasms such as glioblastoma multiforme and anaplastic oligodendrogliomas [410]. According to clinical studies on radiation-induced neoplasms, which is thought to be late-complications of ALL treatment, age of onset and the incidence of high-grade gliomas have an inverse relationship. That is, the younger a patient is at the onset of ALL, the higher the risk for developing high-grade glioma, especially in children under the age of six [32]. Children who have undergone prophylactic CNS radiotherapy are at higher risk of secondary neoplasms, thus clinicians should carefully observe them for a certain period of time after treatment [5].
For low-grade radiation-induced gliomas, the latency period ranges from 1 year to 36 years (Table 1), and in our patient, it was 13 years until the clinical manifestation. While these latency values are higher than those reported in the literature for high-grade gliomas, we can carefully assume that low-grade gliomas takes an indolent course, and takes several more years to develop and manifest clinically [22]. Thus, the latency period of our patient is not accurate since it is not clear when the secondary tumor occurred.
The natural course and clinical outcome of radio-induced low-grade gliomas is still unknown. It has been suggested that radiation-induced secondary neoplasms are likely to possess some different clinical features from their ‘primary’ counterparts [1718], but this suggestion relates to high-grade gliomas arising in younger age patients at atypical sites, and may not be applicable to the pathology of our patient. Regarding the molecular characterization, the LOH 1p and 19q is a common finding with WHO grade II oligodendrogliomas in the general population [25]. It is notable that radiation-induced neoplasms generally exhibit malignant characteristics on histological examination [311]. However, further data from molecular analyses are required, as the present case suggests that this generalization may not be applicable to low-grade radiation-induced oligodendrogliomas, even at the molecular level. Regarding the clinical outcome, observations over a longer follow-up period and studies including more cases are necessary for the scientific community to clarify the biological and clinical properties of such a particular category of tumors, not only to establish the best treatment for these particular subset of patients, but also to improve our understanding of the pathogenetic features of these specific tumors.
The development of a secondary neoplasm in a previously irradiated region is relatively rare when compared with other long-term complications of radiotherapy [16]. Although the outcomes and life expectancy for patients with ALL following treatment have improved significantly in recent years, clinicians should not overlook surveillance of secondary neoplasms. Our case shows that oligodendroglioma should be considered when undertaking differential diagnoses of secondary neoplasms in the brain.
Figures and Tables
Fig. 1
A contrast-enhanced MRI study of the brain shows a mass in a the right insular cortex with a main diameter of 2 cm in the subcortex of the right insula and no post-contrast enhancement.
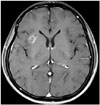
Fig. 2
In the ×400 view, round uniform nuclei, nucleoli, and perinuclear halos are observed, suggestive of a typical oligodendroglioma. The tumor cells are infiltrating into brain parenchyma.
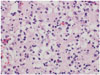
Fig. 3
A: Molecular pathologic results reveals the LOH of chromosomes 1p (ratio-counted tumor nuclei: 50, 1p/1q ratio: 0.64). B: Molecular pathologic results reveals the LOH of chromosomes 19q (ratio-counted tumor nuclei: 50, 19q/19p ratio: 0.65). LOH, loss of heterozygosity.
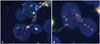
Fig. 4
Molecular analysis reveals positive for the IDH1 mutation at residue 132:pR132H (tumor proportion of the tested tissue: 70%). IDH1, isocitrate dehydrogenase 1.

Fig. 5
A contrast-enhanced MRI study of the brain shows a minimal decrease in the size or extent of the underlying non-enhancing tumor involving the right anterior insular region and possible postopeative or post biopsy tissue defects.
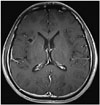
Table 1
Literature review of radiation induced low-grade gliomas

| Case no. | Authors (years) | Age/gender | Primary disease | Radiation dosage (Gy) | Latency (years) | Radiation-induced low-grade glioma |
|---|---|---|---|---|---|---|
| 1 | Jones 1960 [15] | 33/M | Meningioma | 40 | 10 | Astrocytoma |
| 2 | Albert et al. 1966 [1] | 4/M | Tinea capitis | 5–8 | 4 | Astrocytoma |
| 3 | Albert et al. 1966 [1] | 10/M | Tinea capitis | 5–8 | 1 | Astrocytoma |
| 4–5 | Shore et al. [30] | 2/M | Tinea capitis | 3–4 | 5–6 | Astrocytoma |
| 6 | Shore et al. [30] | 8/M | Tinea capitis | 3–4 | 26 | Astrocytoma |
| 7 | Walters [35] | 3/F | ALL | 26.2 | 6 | Astrocytoma |
| 8 | Anderson [6] | 25/F | Medulloblastoma | 42 | 6 | Cerebellar ependymoma |
| 9 | Okamoto [26] | 25/F | Medulloblastoma | 42 | 6 | Infratentorial ependymoma |
| 10–14 | Albo [2] | 3M–2F | ALL | 24 | 6.5 | 4 Astrocytoma+1 ependymoma |
| 15 | Malone [21] | 8/F | ALL | 20 | 3.5 | Infratentorial and spinal astrocytoma |
| 16 | Malone [21] | 19/M | ALL | 25.2 | 4.5 | Astrocytoma |
| 17 | Dierssen [9] | 16/F | Fibrosarcoma | 50 | 11 | Astrocytoma |
| 18 | Dierssen [9] | 15/M | Ear chronic disease | 18 | 11 | Astrocytoma |
| 19–20 | Soffer [31] | 2F | Tinea capitis | ? | ?–36 | Cerebellar+fibrillary astrocytoma |
| 21 | Tsang [33] | 38/M | Pituitary adenoma | 50 | 9 | Astrocytoma |
| 22 | Walter [34] | 2/M | ALL | 24 | 9.2 | Oligodendroglioma |
| 23 | D’Elia [8] | 32/M | ALL | 18 | 22 | Astrocytoma |
| 24 | D’Elia [8] | 34/F | ALL | 24 | 26 | Astrocytoma |
| 25 | Present case | 8 | ALL | 18 | 13 | Oligodendroglioma |
References
1. Albert RE, Omran AR, Brauer EW, et al. Follow-up study of patients treated by x-ray for tinea capitis. Am J Public Health Nations Health. 1966; 56:2114–2120.

2. Albo V, Miller D, Leiken S, Sather H, Hammond D. Nine brain tumours (BT) as a late effect in children “cured” of acute lymphoblastic leukemia (ALL) from a single protocol study (Abstr). Proc Am Soc Clin Oncol. 1985; 4:172.
3. Alexander MJ, DeSalles AA, Tomiyasu U. Multiple radiation-induced intracranial lesions after treatment for pituitary adenoma. Case report. J Neurosurg. 1998; 88:111–115.

4. Alexiou GA. High-grade gliomas in survivors of childhood acute lymphoblastic leukaemia. Childs Nerv Syst. 2009; 25:779. author reply 781-2.

5. Alexiou GA, Moschovi M, Georgoulis G, et al. Anaplastic oligodendrogliomas after treatment of acute lymphoblastic leukemia in children: report of 2 cases. J Neurosurg Pediatr. 2010; 5:179–183.

6. Anderson JR, Treip CS. Radiation-induced intracranial neoplasms. A report of three possible cases. Cancer. 1984; 53:426–429.

7. Borgmann A, Zinn C, Hartmann R, et al. Secondary malignant neoplasms after intensive treatment of relapsed acute lymphoblastic leukaemia in childhood. Eur J Cancer. 2008; 44:257–268.

8. D’Elia A, Melone GA, Brogna C, Formichella A, Santoro A, Salvati M. Radio-induced low-grade glioma: report of two cases and review of the literature. Neurol Sci. 2009; 30:137–141.

9. Dierssen G, Alvarez G, Figols J. Anaplastic astrocytomas associated with previous radiotherapy: report of three cases. Neurosurgery. 1988; 22(6 Pt 1):1095–1097.

10. Hah JO. Anaplastic oligodendroglioma after childhood acute lymphoblastic leukemia: chemotherapy and autologous peripheral blood stem cell transplantation. J Pediatr Hematol Oncol. 2008; 30:764–767.

11. Harrison MJ, Wolfe DE, Lau TS, Mitnick RJ, Sachdev VP. Radiation-induced meningiomas: experience at the Mount Sinai Hospital and review of the literature. J Neurosurg. 1991; 75:564–574.

12. Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007; 99:300–308.

13. Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007; 297:1207–1215.

14. Ishida Y, Maeda M, Urayama KY, et al. Secondary cancers among children with acute lymphoblastic leukaemia treated by the Tokyo Children’s Cancer Study Group protocols: a retrospective cohort study. Br J Haematol. 2014; 164:101–112.

15. Jones A. Supervoltage X-ray therapy of intracranial tumours. Ann R Coll Surg Engl. 1960; 27:310–354.
16. Kaschten B, Flandroy P, Reznik M, Hainaut H, Stevenaert A. Radiation-induced gliosarcoma. Case report and review of the literature. J Neurosurg. 1995; 83:154–162.
17. Kitanaka C, Shitara N, Nakagomi T, et al. Postradiation astrocytoma. Report of two cases. J Neurosurg. 1989; 70:469–474.
18. Klériga E, Sher JH, Nallainathan SK, Stein SC, Sacher M. Development of cerebellar malignant astrocytoma at site of a medulloblastoma treated 11 years earlier. Case report. J Neurosurg. 1978; 49:445–449.

19. Löning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Münster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood. 2000; 95:2770–2775.
20. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.

21. Malone M, Lumley H, Erdohazi M. Astrocytoma as a second malignancy in patients with acute lymphoblastic leukemia. Cancer. 1986; 57:1979–1985.

22. Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003; 53:524–528.

23. Neglia JP, Meadows AT, Robison LL, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med. 1991; 325:1330–1336.

24. Nishio S, Morioka T, Inamura T, et al. Radiation-induced brain tumours: potential late complications of radiation therapy for brain tumours. Acta Neurochir (Wien). 1998; 140:763–770.

26. Okamoto S, Handa H, Yamashita J, Tokuriki Y, Abe M. Post-irradiation brain tumors. Neurol Med Chir (Tokyo). 1985; 25:528–533.

27. Park KJ, Kang SH, Lee HG, Chung YG. Olfactory neuroblastoma following treatment for pituitary adenoma. J Neurooncol. 2008; 90:237–241.

28. Relling MV, Rubnitz JE, Rivera GK, et al. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999; 354:34–39.

29. Ron E, Modan B, Boice JD Jr, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988; 319:1033–1039.

30. Shore RE, Albert RE, Pasternack BS. Follow-up study of patients treated by X-ray epilation for Tinea capitis; resurvey of post-treatment illness and mortality experience. Arch Environ Health. 1976; 31:21–28.
31. Soffer D, Gomori JM, Pomeranz S, Siegal T. Gliomas following low-dose irradiation to the head report of three cases. J Neurooncol. 1990; 8:67–72.

32. Tanriover N, Ulu MO, Sar M, Uzan M. Anaplastic oligoastrocytoma: previous treatment as a possible cause in a child with acute lymphoblastic leukemia. Childs Nerv Syst. 2007; 23:469–473.

33. Tsang RW, Laperriere NJ, Simpson WJ, Brierley J, Panzarella T, Smyth HS. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer. 1993; 72:2227–2233.

34. Walter AW, Hancock ML, Pui CH, et al. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J Clin Oncol. 1998; 16:3761–3767.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download