Abstract
Uterine adenosarcoma (AS) are rare tumors and have more favorable outcomes than the aggressive uterine carcinosarcomas. Uterine adenosarcoma with sarcomatous overgrowth (ASSO) is a variant form of AS and exhibits aggressive growth of tumor and the prognosis is relatively poor compared with typical AS. Usually patterns of metastasis have been known to behave like endometrial carcinoma and spread through the lymphatics. Brain metastasis from uterine AS is extremely rare. Herein, we report a case of successfully surgically removed solitary brain metastasis without any extracranial recurrence from uterine ASSO after 4 years of primary treatment.
Malignant adenosarcoma (AS) could originate anywhere from female genital organs and these are particularly rare sarcomas that accounts for less than 10% of uterine sarcomas and about 1% of female genital tract malignancies and 3–7% of uterine cancers [123]. It was first used for a distinctive uterine tumor characterized by a mostly low-grade malignant stromal component and a benign glandular epithelial component [45].
Furthermore, this tumor differ histologically from other sarcomas that they contain both epithelial and stromal component [6]. Uterine AS has been considered to be indolent tumors although recurrence rates have been reported in 26–30% [7]. Uterine adenosarcoma with sarcomatous overgrowth (ASSO) is a variant form of AS and exhibits aggressive growth of tumor and the prognosis is relatively poor compared with typical AS [18].
Brain metastasis from solid tumor is rare and predicts a grave outcome. Overall survival from time of metastasis to death varies considerably, from 1 month to 20 months with some patients showing modest responses to radiotherapy. In some solid tumors, patients with a good performance status, a well-controlled extracranial disease and a solitary brain lesion are considered for surgical resection. Central nervous system involvement in uterine AS is a very rare and usually late fatal complication with a very short survival [910].
Herein, we present the interesting case of 55-year-old female patient who was diagnosed as brain metastasis and underwent complete surgical resection for her solitary metastatic disease, 4 years after the primary uterine AS treatment of radical resection and adjuvant chemotherapy. We also review the literature on uterine AS with a focus on brain metastasis and discuss treatment strategy.
A 51-year-old female admitted on March, due to large amount of vaginal bleeding. On transvaginal ultrasound, uterine was woman's feast sized, globular 3 cm sized myoma were identified in anterior lower uterine and both ovaries were normal. Biopsy under the hysteroscopy was done and showed uterine AS with histological high grade with stromal overgrowth, 30–40 brisk mitotic figures in 10 high power fields, no heterologous component, microscopically tumor cell necrosis present. Clinical staging work up was performed including CT scan and Positron Emission Tomography CT and the disease was confined to the pelvis.
She received total abdominal hysterectomy, bilateral salpingo-oophorectomy and pelvic lymph node dissection on explorative laparotomy. On gross examination, the uterus is distorted due to bulging intramural mass. There was no evidence of peritoneal seeding at exploration or any lymph node involvement from resection. Pathologic diagnosis was ASSO, high grade (Fig. 2). Vascular invasion and lymph nodes involvement were not showed. The patient had received six cycles of paclitaxel and ifosfomide chemotherapy as adjuvant treatment and no evidence of disease was noted on follow-up imaging.
About 4-year later, she presented to the emergency room with right-sided weakness for 2 hours. On physical examination the patient appeared acutely ill and vital sign was normal range. Motor grade in the right upper grade was IV, right lower grade was III and left side was V. sensory is intact, her cranial nerve examination was normal.
Brain MRI revealed newly appeared well-enhancing lobulated mass with extensive surrounding edema in left frontal lobe measuring 3.8×2.8 cm and midline shifting to right side (Fig. 1). This lesion was metastasis more likely. CT scan of chest and abdomen/pelvis did not show any evidence of systemic disease. Dexamethasone treatment was started with an improvement of neurologic symptoms. Craniotomy with radical tumor excision in left medial frontal lobe was done. Tissue from the tumor was sampled, and an intraoperative frozen-section diagnosis of metastatic carcinoma was rendered. Pathologic diagnosis was metastatic sarcoma, consistent with uterine AS. Sarcomatous component was dominant in brain metastatic AS lesion compared to primary tumor (Fig. 3). Postoperative brain MRI demonstrated a gross total resection with no evidence of residual enhancing mass and her right upper and lower limb motor were recovered to grade V and the patient was discharged. Although postsurgical additional radiation therapy to brain was recommend, our case that underwent brain surgery refused subsequent radiation and chose close observation with no immediate additional active treatment. At a follow-up 12 months, the patient is still alive in good clinical conditions except mild neurologic deficit after resection and there was no evidence of tumor recurrence.
Uterine AS is a rare tumor and contain a variety of epithelial and connective tissue elements and have been reported since when Clement et al. [4] described first case of a uterine AS in 1974 and reviewed by the same authors later [7]. This tumor is uncommon variant of mixed Mullerian tumors of the uterus characterized by a benign glandular component intermingled with sarcomatous stroma [11]. After then, many clinical and pathological studies have been conducted and now support the concept that the majority of these neoplasm are monoclonal in origin, being derived from single stem cells of the Mullerian epithelium with sarcomatous elements being a result of metaplasia or dedifferentiation [12]. Sarcomatous overgrowth occurs in 8–65% of AS and has a malignant sarcomatous component of high grade differentiation with severe atypia and many mitosis that constitutes more than 25% of the tumor [1213]
Uterine AS are differentiated from uterine carcinosarcomas by presence of a benign looking epithelial component and a low grade sarcomatous components and have a more favorable prognosis than other uterine sarcomas [78]. However, uterine ASSO is a more aggressive tumor than usual AS that has rapid growth with direct tumor extension similar to other high-grade uterine sarcomas [1415]. Recurrences in uterine AS occur in more than a quarter of patients after primary tumor treatment. Even in early-stage disease, rates of recurrence are relatively high. Local failure including intra-abdomen lesion have been reported more often in patients with uterine AS than distant metastatic disease and additionally patterns of metastasis have been known to behave like endometrial carcinoma and spread through the lymphatics. Mortality related to this cancer is usually secondary locally recurrent pelvic and/or abdominal disease rather than other visceral metastasis [14]. Unfavorable prognostic factors include presence of heterologous elements, necrosis, high mitotic rate, vascular invasion and extrauterine spread. Sarcomatoid overgrowth like our case is also important unfavorable histological factor.
Cerebral metastasis is a rare manifestation of endometrial carcinoma occurring in 0.3–0.9% of patients usually late within the course of the disease. And brain metastasis from an AS of the uterus is more extremely rare. To our best knowledge, it's only a few similar cases have been reported in the literature [59161718] (Table 1). Our case is unique because there was no evidence of metastasis except single brain metastatic lesion pathologically confirmed as ASSO from uterus.
However, compared to other reported cases, her brain metastasis in our case was detected without any other site metastasis and furthermore, the recurrent disease in brain was documented with solitary mass and treated successfully with surgical resection. Almost of reported cases exhibited grave prognosis with a very short survival of 2–3 months. The other case report from Kim et al. [18], they also tried to resect isolated cerebellar metastasis, however, unfortunately brain lesion showed rapid local recurrence only in a month after brain surgery. But in our case, the patient has not showed recurrence in both intracranial and extracranial area.
Optimal treatment of this kind of tumor is still unclear and no definite treatment guideline has been issued [1]. In this case we completely resected the mass, and after resection the patient could get another chance of disease control until now in this aggressive tumor which has been considered a highly malignant tumor because of its rapid recurrence. Our case suggests that aggressive surgical resection in selected patients with brain metastasis from AS should be considered. In conclusion, brain metastasis from a uterine ASSO is extremely rare and typically has shown a highly aggressive clinical course with a short survival. We report an interesting case that developed solitary brain metastasis without extracranial metastasis and surgically removed successfully from ASSO.
Figures and Tables
Fig. 1
MRI findings of a solitary brain metastasis from uterine carcinosarcoma. A solitary, necrotic lesion in the leftt central region with a cerebral surrounding edema resulting midline shifting (brain MRI T1 weighted with gadolinium-enhancement image).
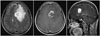
Fig. 2
Uterine adenosarcoma with sarcomatoid epithelial (A) and stromal components are exist and showed sarcomatoid overgrowth (B).
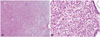
Fig. 3
A and B: Brain metastasis area exhibits sarcomatous overgrowth having stromal cells displaying obvious cytologic atypia and increased mitotic figures. In immunohistochemical staining, CD10 was positive in glial lesion and negative in tumor lesion (C) and CD34 was positive (D).
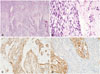
Table 1
Summary of the reported patients with CNS metastasis from uterine carcinosarcoma/adenosarcoma

| Author/year | Age | Metastases lesion | Treatment | Survival |
|---|---|---|---|---|
| Iqbal et al. [16] | 51 | Left frontal | Surgery+radiation | 25 months |
| Cormio et al. [17] | 48 | Spinal cord compression, multiple brain | Surgery | 1 month |
| N’ kanza et al. (2005) [9] | 61 | Parietal & cerebellum | Autopsy only | 2 months |
| Kim et al. [18] | 57 | Cerebellum | Surgery | 2 months |
| Daskalaki et al. [5] | 75 | Multiple | No treatment | 4 days |
| Our case | 51 | Left frontal | Surgery | 12+months |
References
1. Carroll A, Ramirez PT, Westin SN, et al. Uterine adenosarcoma: an analysis on management, outcomes, and risk factors for recurrence. Gynecol Oncol. 2014; 135:455–461.

2. D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010; 116:131–139.
3. Bernard B, Clarke BA, Malowany JI, et al. Uterine adenosarcomas: a dual-institution update on staging, prognosis and survival. Gynecol Oncol. 2013; 131:634–639.

4. Clement PB, Scully RE. Müllerian adenosarcoma of the uterus. A clinicopathologic analysis of ten cases of a distinctive type of müllerian mixed tumor. Cancer. 1974; 34:1138–1149.

5. Daskalaki A, Xenaki S, Athanasakis E, Chrysos E, Chalkiadakis G. Advanced mesodermal (Müllerian) adenosarcoma of the ovary: metastases to the lungs, mouth, and brain. Case Rep Surg. 2015; 12. 30. DOI: 10.1155/2015/403431.

6. Sinha A, Phukan JP, Sengupta S, Guha P. Mullerian adenosarcoma of uterus with sarcomatous overgrowth and heterologous component associated with stromal deposit in omentum: a case report and review of the literature. Case Rep Med. 2012; Aug. 16. DOI: 10.1155/2012/820378.

7. Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol. 1990; 21:363–381.

8. Kaku T, Silverberg SG, Major FJ, Miller A, Fetter B, Brady MF. Adenosarcoma of the uterus: a Gynecologic Oncology Group clinicopathologic study of 31 cases. Int J Gynecol Pathol. 1992; 11:75–88.
9. N'Kanza AL, Jobanputra S, Farmer P, Lovecchio J, Yelon JA, Rudloff U. Central nervous system involvement from malignant mixed Müllerian tumor (MMMT) of the uterus. Arch Gynecol Obstet. 2005; 273:63–68.
10. Inthasorn P, Beale P, Dalrymple C, Carter J. Malignant mixed mullerian tumour of the ovary: prognostic factor and response of adjuvant platinum-based chemotherapy. Aust N Z J Obstet Gynaecol. 2003; 43:61–64.

11. Arici DS, Aker H, Yildiz E, Tasyurt A. Mullerian adenosarcoma of the uterus associated with tamoxifen therapy. Arch Gynecol Obstet. 2000; 264:105–107.

12. Piscuoglio S, Burke KA, Ng CK, et al. Uterine adenosarcomas are mesenchymal neoplasms. J Pathol. 2016; 238:381–388.

13. Gallardo A, Prat J. Mullerian adenosarcoma: a clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am J Surg Pathol. 2009; 33:278–288.
14. Major FJ, Blessing JA, Silverberg SG. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993; 71:4 Suppl. 1702–1709.

15. Krivak TC, Seidman JD, McBroom JW, MacKoul PJ, Aye LM, Rose GS. Uterine adenosarcoma with sarcomatous overgrowth versus uterine carcinosarcoma: comparison of treatment and survival. Gynecol Oncol. 2001; 83:89–94.

16. Iqbal JB, Ironside JW. Cerebral metastasis from a malignant mixed müllerian tumour of the uterus. Histopathology. 1993; 23:277–279.

17. Cormio G, Colamaria A, Di Vagno G, Pierangeli E, Vailati G, Selvaggi L. Central nervous system involvement secondary to metastatic mixed müllerian tumor of the uterus. Gynecol Obstet Invest. 1997; 44:214–216.

18. Kim JK, Lee SK, Myong NH, Kang YD. Biopsy-proven cerebellar metastasis from a malignant mixed mullerian tumor (MMMT) of the uterus: case report. Eur J Gynaecol Oncol. 2009; 30:196–198.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download