Abstract
A 63-year-old man complained of intermittent motor weakness of his arm. The magnetic resonance image (MRI) of his brain displayed a high signal lesion in right cingulate gyrus on T2 weighted image. One year later, he showed a stuporous mental status with repeated seizures, and the follow-up brain MRI showed heterogeneously enhanced mass associated with bleeding. He was treated with surgery and radiotherapy for secondary glioblastomas in right cingulate gyrus. One year more later, a mass recurred on the left frontal base, and gliosarcoma was diagnosed. After tumor resection, ventriculoperitoneal shunt, chemotherapy, and re-radiation therapy, all brain lesions were stable. Fourteen months after the diagnosis of gliosarcoma, he complained of dyspnea and back pain. Torso positron emission tomography/computed tomography revealed multiple metastatic lesions in both lungs, pericardium, pleura, liver, lymph nodes, and bones, and metastatic gliosarcoma was diagnosed. One month later, the patient died because of the systemic metastases. We present an unusual case of secondary gliosarcoma with stable brain lesions and extensive systemic metastases.
Gliosarcomas are uncommon malignant brain tumor, composed of mixed gliomatous and sarcomatous component [12]. Gliosarcoma is a rare variant of glioblastoma and comprise 1.8–8% of glioblastoma [3]. Gliosarcoma is a very aggressive tumor, and the prognosis is similar to glioblastoma [4]. The invasion of dura and extra-cranial metastase are more common in gliosarcoma than glioblastoma [5]. The extra-cranial metastases are located in lungs, liver, and lymph nodes, which is related with poor prognosis [6]. The distant metastases are mostly associated with a recurrence of gliosarcoma on brain. Here, we report a rare case of secondary gliosarcoma with stable brain lesions and extensive systemic metastases to the lungs, pleura, pericardium, liver, lymph nodes, and bones.
A 62-year-old man complained of intermittent motor weakness of his left arm. Neurologic examination showed no specific motor weakness. Tthe initial T2-weighted magnetic resonance images (MRI) of his brain displayed a high-signal lesion in right cingulate gyrus (Fig. 1A). The radiologic follow-up was recommended. He was lost to follow-up.
One year later, he was admitted to the hospital in a stupor with repeated seizures. The follow-up brain MRI showed heterogeneously enhanced mass associated with bleeding in right cingulate gyrus (Fig. 1B, C, D). The tumor was totally resected (Fig. 1E), and the patient was diagnosed with glioblastoma (Fig. 2A, B). Radiotherapy of 59.4 Gy was administered without chemotherapy because of the drowsy mental status and poor general condition.
One year more later, the patient developed a gait disturbance and memory impairment. Brain MRI showed a homogenous enhanced lesion in the left frontal base, associated with hydrocephalus, and there was no recurrence of the previously treated lesion (Fig. 1F, G, H). The mass was totally resected, and a ventriculoperitoneal shunt was placed (Fig. 1I). The pathology showed a biphasic pattern with areas of gliomatous and sarcomatous differentiation. The gliomatous component showed immunoreactivity for glial fibrillary acid protein, and the sarcomatous portion had immunopositivity for vimentin and nestin (Fig. 2C-F). The morphologic and immunohistochemical features suggested a gliosarcoma.
Chemotherapy was administered with 2 cycles of temozolomide. The mass continued to progress (Fig. 1J). Re-radiation therapy of 50 Gy was administered to the recurrent lesion on the left frontal base. After radiotherapy, all brain lesions were stable (Fig. 1K).
Fourteen months after the diagnosis of secondary gliosarcoma, he complained of dyspnea and back pain without deterioration of neurologic status. Chest computed tomography (CT) showed pulmonary metastases, pleural effusion, and pericardial metastases. Torso positron emission tomography/CT revealed multiple metastatic lesions in both the lungs, pericardium, left pleura, right mediastinal pleura, liver, lymph nodes, and bones (Fig. 1L). A percutaneous liver biopsy was performed. Pathology revealed a sarcomatous component with immunopositivity for vimentin and nestin, and the patient was diagnosed with metastatic gliosarcoma (Fig. 2G, H). The pleural effusion was enlarged, and dyspnea was aggravated. One month after the onset of dyspnea and back pain, the patient died.
Gliosarcomas are uncommon malignant central nervous system neoplasms that can develop de novo or subsequent to a diagnosis of glioblastoma. Radiation-induced gliosarcomas, called secondary gliosarcomas, arise after intracranial radiation for malignant gliomas. Dural invasion is frequently seen in gliosarcomas with homogenous contrast enhancement and less edema compared to glioblastoma. The tumor is predominantly located in the temporal lobe, is often located superficially, presents surgically as a firm lesion adherent to the meninges, and has occasionally been mistaken for meningioma during surgery. Pathologically, gliosarcoma is defined as a glioblastoma variant consisting of an admixture of gliomatous and sarcomatous components, and presents in around 2% of glioblastoma cases [12]. The glial component is pleomorphic astrocytic cells with immunopositivity for glial fibrillary acidic protein, and the sarcomatous component shows malignant changes of spindle cells with immunopositivity for vimentin and S-100. The prognosis for gliosarcoma is similar to that of glioblastoma, with a mean survival time ranging from 6 to 14.8 months.
Gliosarcoma may show extra-cranial metastases, although rarely, with the most common sites being the lungs, liver, and lymph nodes [7]. The intrinsic biological barriers to prevent systemic metastases from the brain are the absence of a lymphatic system within the brain and spinal cord, dense dura, and lack of a nurturing stroma. Repeated surgical resection and ventricular shunting can cause dissemination of tumor cells into the blood stream. Compared to other central nervous system malignant tumors, including glioblastoma, gliosarcoma has a greater propensity for extracranial metastases. When compared to glioblastoma, gliosarcoma is associated with a higher likelihood for extracranial dissemination. The presence of a sarcomatous component may result in a high propensity to metastasize. Extracranial metastases from glioblastoma have been reported in 0.2–1.2% of cases versus 11% for gliosarcoma [67]. Hematogenous metastases may result from invasion of the intratumoral vessels and transdural penetration into the dural sinuses caused by craniotomy procedures or cranial irradiation. The most common sites are the lungs, liver, and lymph nodes, and there are reports of metastatic foci in the spleen, adrenal glands, kidneys, oral mucosa, skin, bone marrow, skull, ribs, and spine.
Patients with metastases to the neck and liver showed a better prognosis than those with lung metastases. However, the presence of distant metastases is commonly associated with a dismal prognosis. Evaluation for extra-cranial disease may be necessary in gliosarcomas at diagnosis, and surveillance is required because of the propensity for hematogenous spread. In most cases, the distant metastases are associated with a recurrence of gliosarcoma. In some cases, a limited number of extracranial metastases were not associated with a recurrence.
In conclusion, this is an unusual case of secondary gliosarcoma with stable brain lesions and extensive systemic metastases to the lungs, pleura, pericardium, liver, lymph nodes, and bones.
Figures and Tables
Fig. 1
Radiologic findings of glioblastoma and gliosarcoma. A: Initial T2-weighted magnetic resonance images (MRI) of the brain displays a high-signal lesion in right cingulate gyru. B, C, and D: One year later, follow-up MRI shows a heterogeneously enhanced mass associated with bleeding in right cingulate gyrus on axial (B), sagittal (C), and coronal (D) images of enhanced T1-weighted image. E: On brain computed tomography (CT), the tumor was totally resected. F: Brain MRI shows a homogenous enhanced lesion in the left frontal base on axial view of enhanced T1-weighted image. G: Ventricular enlargement with periventricular lucency was shown on T2-weighted MR image. H: There was no recurred lesion of right cingulate gyrus on enhanced T1-weighted sagittal image. I: Brain CT shows that the mass was totally resected, and a ventriculoperitoneal shunt was placed. J: After surgery and chemotherapy, the mass of left frontal base was recurred on axial view of enhanced T1-weighted image. K: After radiotherapy, the mass of left frontal base was stable on axial view of enhanced T1-weighted image. L: Torso positron emission tomography/CT shows multiple metastatic lesions in both the lungs, pericardium, left pleura, right mediastinal pleura, liver, lymph nodes, and bones.
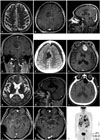
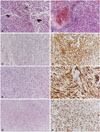
Fig. 2
Pathologic findings of glioblastoma and gliosarcoma within brain and liver. Glioblastoma of brain; pleomorphic neoplastic cells with endothelial proliferation (A) and pseudopalisading necrosis (B) (original magnification, ×100; hematoxylin and eosin staining). Gliosarcoma of brain; the glial component of pleomorphic astrocytic cells (C, original magnification, ×100; hematoxylin and eosin staining), immunopositivity for glial fibrillary acidic protein (D, original magnification, ×200), sarcomatous component of spindle cells (E, original magnification, ×100; hematoxylin and eosin staining) and immunopositivity for nestin (F, original magnification, ×200). Gliosarcoma of liver; sarcomatous component of spindle cells (G, original magnification, ×100; hematoxylin and eosin staining) and immunopositivity for nestin (H, original magnification, ×200).
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014R1A1A1004469).
References
1. Ben Nsir A, Thai QA, Kassar AZ, Ben Said I, Jemel H. Primary cerebellar gliosarcoma with extracranial metastases: an orphan differential diagnosis. World Neurosurg. 2015; 84:2076.e13-7.

2. Rapp M, Felsberg J, Sorg RV, Gerharz CD, Sabel M. Case report: extracranial metastasis from gliosarcoma--the influence of immune system. Br J Neurosurg. 2011; 25:286–288.

3. Andaloussi-Saghir K, Oukabli M, El Marjany M, Sifat H, Hadadi K, Mansouri H. Secondary gliosarcoma after the treatment of primary glioblastoma multiforme. N Am J Med Sci. 2011; 3:527–530.

4. Rizvi S, Asghar AH, Mehboob J. Gliosarcoma: a rare variant of glioblastoma multiforme. J Pak Med Assoc. 2010; 60:773–775.
5. Mason A, Villavicencio AT, Nelson EL, Forsythe RC, Burneikiene S. Post-treatment gliosarcoma extension into the pterygomaxillary fossa: literature review and case report. Cureus. 2016; 8:e700.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download