Abstract
Giant cell tumors are benign but locally invasive and frequently recur. Giant cell tumors of the skull are extremely rare. A patient underwent a surgery to remove a tumor, but the tumor recurred. Additionally, the patient developed multiple aneurysms. The patient underwent total tumor resection and trapping for the aneurysms, followed by radiotherapy. We report this rare case and suggest some possibilities for treating tumor growth combined with aneurysm development.
Giant cell tumors (GCTs) of the bone originate from bone marrow connective tissue. The majority of cases occur in the epiphysis of long bones. GCT is rare, representing 3–7% of all bony tumors [123], and less than 2% of cases involve the skull [4]. GCTs of the skull occur most frequently in the sphenoid bone [356789] and the temporal bone [5610111213141516]. GCT is a benign neoplasm, but it can be locally aggressive [61718] and has high rates of local recurrence [19]. Here, the authors present a case of GCT involving the temporal bone in a patient who experienced recurrence with dural invasion and newly developedcerebral aneurysms within 9 months of the first surgical resection.
A 37-year-old man presented with dizziness and left side hearing impairment that had been present for about 4 years. On physical examination, the patient was healthy in appearance with no apparent abnormalities of the skull or scalp. Pre-operative magnetic resonance imaging (MRI) showed an approximately 3-cm mass at the petrous part of left temporal bone (Fig. 1). The mass was destructing the temporal bone and invading the posterior semicircular canal and middle ear cavity. Additionally, the mass bulged into the adjacent cerebellopontine angle (CPA) cistern and extended to the left jugular foramen.
The patient underwent a mastoidectomy at another institute. According to the operative description written by the surgeon, an infra-temporal fossa approach type A was used for tumor removal. The malleus and incus had eroded so they were removed. The tumor extended to the petrosal apex and invaded the cochlea and semicircular canal. During the procedure, the dura of the petrosal apex area was damaged, causing cerebrospinal fluid leakage.
During the acute post-operative phase after the first surgery, no other neurological deficit was found except left side hearing impairment generated by the unavoidable injury of the vestibulocochlear system. Post-operative MRI showed near total removal of the tumor (Fig. 1).
Follow-up MRI was performed 9 months after the surgery. On the post-operative MRI, the remnant tumor had regrown and formed a mass at the CPA that measured about 2.5×1.5 cm, and the tumor had invaded the dura membrane forming perilesional parenchymal edema in the left cerebellum (Fig. 2).
Cerebral angiography was performed to examine pre-operative vessels and tumor embolization. On angiography, there were newly noted multiple, beaded patterns distal to the anterior inferior cerebellar artery (AICA) aneurysms adjacent to tumor (Fig. 2). The aneurysms were fusiform and located distally to the AICA. The AICA and posterior inferior cerebellar artery (PICA) were anastomosed, and therefore, endovascular treatment for these aneurysms was thought to be risky.
On admission, there was no specific neurological deficit, except for the left hearing impairment. Using a lateral suboccipital craniectomy approach, the incision was made on the previous surgical wound. Severe adhesion occurred between the tumor and the vessels and nerves. The tumor mass was very bloody and fragile. Tumor removal was performed from the mastoid wall, which was considered to be the tumor origin, to the dural invasion (Fig. 3). After total removal of the tumor, three consecutive distal AICA aneurysms were found. Clipping of the aneurysms was unfavorable because all aneurysms were fusiform and had fragile vessel walls. On pre-operative angiography, we found well-developed collaterals between the distal AICA and PICA, so we trapped the most proximal and distal portions of the aneurysm and resected the middle portion. The histopathological findings of the tumor revealed a characteristic storiform pattern, consisting of spindled or ovoid-shaped cells showing atypia and mitosis, and many multi-nucleated giant cells in bone fragments and the brain parenchyma.
On post-operative MRI, the tumor was totally removed (Fig. 2). Because of the rapid tumor recurrence after the first surgery, radiation therapy was performed after the second surgery with total dose of 4,300 cGy over 20 treatments.
GCTs account for approximately 4–9.5% of all skeletal tumors and 18–23% of benign tumors [1]. This tumor develops by endochondral ossification, and most GCTs (70–90%) occur at the epiphyses of long bones.
Only 1% of GCTs present in the skull [2021], with the most common cranial sites being the sphenoid and temporal bones. Because the sphenoid bone and petromastoid portions of the temporal bone arise from endochondral ossification in the skull, this can explain why GCTs in the skull are mostly found in the sphenoid and temporal bones [622]. In this report, the tumor seemed to originate from the mastoid wall of the temporal bone and extend to the semicircular canal. Common symptoms of patients with GCTs are headaches and palpable masses, but in this case, the patient complained of dizziness and hearing impairment due to tumor invasion of the semicircular canal.
The tumor was almost totally removed, but recurred within 9 months. The recurred tumor was located at the CPA. The tumor recurred from its residual tissue at the CPA likely because the mastoid bone was removed during the first surgery. Despite the benign pathologic findings of the regrown tumor, there was parenchymal brain invasion that was not seen during the first surgery. There was also semicircular canal invasion of the tumor on MRI before the first surgery. According to previous reports, there have been some cases of parenchymal invasion of GCTs with malignant pathologic findings [1923]. Despite generally being benign, the natural history of GCTs is locally aggressive, and they recur at a high rate (10–20%) [24].
In addition, multiple new aneurysms were noted on follow-up MRI, which were not found during pre-operative evaluation. Several hypotheses have been proposed to explain the possible association between tumors and aneurysms. First, an increase in the directional blood flow due to a higher blood supply to a tumor may induce secondary changes in the arterial wall and thus facilitate the formation of aneurysms [2526]. In our opinion, 9 months is a short time for vessel walls to change in response to blood flow. Second, the role of factors in promoting tumor vascularization is a direct predisposing factorfor aneurysm formation [2627]. Additionally, intracranial tumor surgery may also cause traumatic aneurysms [2829]. In this case, however, the chances of trauma seem to be very low because the first surgery was confined to the mastoid bone. Finally, for aneurysms that are within or adjacent to a brain tumor, tumor invasion of the vessel wall points to a causal relationship between the growth of the tumor and the development of the aneurysm [303132]. In this case, the aneurysm wall was attached to the tumor mass. Considering that GCT is benign but locally invasive, this finding might support the hypothesis of aneurysm wall invasion by the tumor. Even though we could not get an aneurysm specimen and could not confirm the pathology, the aneurysm wall would be very fragile. Therefore,careful consideration is necessary in making a decision, and intra-operative aneurysm treatment may be safer than endovascular treatment. In this case, the multiple aneurysms were attached to anterior side of the tumor mass so the aneurysms could only be exposed after the tumor was removed. Therefore, we approached this using lateral suboccipital craniectomy and secured the parent artery of the aneurysms before performing tumor removal.
Surgery is the only effective treatment for GCTs of the skull [10]. However, GCTs are locally aggressive and frequently recur. Therefore, radiation therapy could be recommended. However, the role of radiation therapy is controversial because sarcomatous degeneration is more frequent after irradiation [18]. Radiation treatment is, as a rule, restricted to inoperable tumors or patients without radical surgeries [3334]. In this case, the remnant tumor after the first surgery regrew within 9 months even larger than the original mass. Because of this rapid growing tumor, the patient opted for postoperative radiotherapy.
In conclusion, we report this rare case of GCT of the skull treated by complete surgical removal followed by radiotherapy. Recurred or regrown GCT should be evaluated for different pathological characters, such as sarcomatous degeneration or adjacent tissue invasion.
Figures and Tables
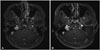 | Fig. 1Comparison of MR images before and after first operation. A: Pre-operative MRI shows a tumor located at left cerebellopontine angle. B: After the first surgery, the tumor was removed. |
References
1. Bertoni F, Unni KK, Beabout JW, Ebersold MJ. Giant cell tumor of the skull. Cancer. 1992; 70:1124–1132.

2. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. Cancer; 25:1061–1070.

3. Gupta R, Mohindra S, Mahore A, Mathuriya SN, Radotra BD. Giant cell tumour of the clivus. Br J Neurosurg. 2008; 22:447–449.

4. Bibas-Bonet H, Fauze RA, Lavado MG, Páez RO, Nieman J. Garcin syndrome resulting from a giant cell tumor of the skull base in a child. Pediatr Neurol. 2003; 28:392–395.

5. Kashiwagi N, Hirabuki N, Andou K, et al. MRI and CT findings of the giant cell tumors of the skull; five cases and a review of the literature. Eur J Radiol. 2006; 58:435–443.

6. Pai SB, Lalitha RM, Prasad K, Rao SG, Harish K. Giant cell tumor of the temporal bone--a case report. BMC Ear Nose Throat Disord. 2005; 5:8.
7. Rimmelin A, Roth T, George B, Dias P, Clouet PL, Dietemann JL. Giant-cell tumour of the sphenoid bone: case report. Neuroradiology. 1996; 38:650–653.

8. Yamamoto M, Fukushima T, Sakamoto S, Tomonaga M. Giant cell tumor of the sphenoid bone: long-term follow-up of two cases after chemotherapy. Surg Neurol. 1998; 49:547–552.

9. Zorlu F, Selek U, Soylemezoglu F, Oge K. Malignant giant cell tumor of the skull base originating from clivus and sphenoid bone. J Neurooncol. 2006; 76:149–152.

10. Elder JB, Berry C, Gonzalez-Gomez I, Kreger MD, McComb JG. Giant cell tumor of the skull in pediatric patients. J Neurosurg. 2007; 107:Suppl 1. 69–74.

11. Lee MY, Lee EJ. Giant cell tumor of the petrous temporal bone with direct invasion into the middle ear. J Craniofac Surg. 2006; 17:797–800.

12. Matsushige T, Nakaoka M, Yahara K, et al. Giant cell tumor of the temporal bone with intratumoral hemorrhage. J Clin Neurosci. 2008; 15:923–927.

13. Coumbaras M, Pierot L, Felgeres AA, Boulin A, Gaillard S, Derome PJ. Giant-cell tumour involving the cranial vault: imaging and treatment. Neuroradiology. 1999; 41:826–828.

14. Ortore RP, Bovo R, Ciorba A, Ceruti S, Martini A. Giant cell granuloma of the temporal bone: a case report. B-ENT. 2008; 4:45–48.
15. Pelaz AC, Llorente Pendás JL, Rodrigo Tapia JP, Suárez Nieto C. Giant cell tumor of the greater wing of the sphenoid: an unusual presentation. J Craniofac Surg. 2008; 19:822–826.
16. Sharma RR, Verma A, Pawar SJ, et al. Pediatric giant cell granuloma of the temporal bone: a case report and brief review of the literature. J Clin Neurosci. 2002; 9:459–462.

17. Kamoshima Y, Sawamura Y, Imai T, Furukawa H, Kubota K, Houkin K. Giant cell tumor of the frontal bone in a girl: case report. Neurol Med Chir (Tokyo). 2011; 51:798–800.
18. Prasad SC, Piccirillo E, Nuseir A, et al. Giant cell tumors of the skull base: case series and current concepts. Audiol Neurootol. 2014; 19:12–21.

19. Wong RH, Thakral B, Watkin WG, Merrell R, Wong AK, Farhat HI. Intracranial, intra-axial metastatic giant cell tumor of bone: case report and review of literature. Clin Neurol Neurosurg. 2014; 117:40–43.

21. Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics. 2001; 21:1283–1309.

22. Lee JA, Bank WO, Gonzalez-Melendez M, Olan WJ, Tabbara SO. Giant cell tumor of the skull. Radiographics. 1998; 18:1295–1302.

23. Oh SJ, Kim KM, Hong TH, Park WC, Kim JS, Jung SS. Giant cell malignant fibrous histiocytoma of the breast: a case report. J Korean Med Sci. 2004; 19:477–480.

24. Saiz P, Virkus W, Piasecki P, Templeton A, Shott S, Gitelis S. Results of giant cell tumor of bone treated with intralesional excision. Clin Orthop Relat Res. 2004; (424):221–226.

25. Delfini R, Domenicucci M, Ferrari M. Association of intracranial meningiomas and aneurysms. J Neurosurg Sci. 1990; 34:51–56.
26. Pia HW, Obrador S, Martin JG. Association of brain tumours and arterial intracranial aneurysms. Acta Neurochir (Wien). 1972; 27:189–204.

27. Pant B, Arita K, Kurisu K, Tominaga A, Eguchi K, Uozumi T. Incidence of intracranial aneurysm associated with pituitary adenoma. Neurosurg Rev. 1997; 20:13–17.

28. Dunn IF, Woodworth GF, Siddiqui AH, et al. Traumatic pericallosal artery aneurysm: a rare complication of transcallosal surgery. J Neurosurg. 2007; 106:2 Suppl. 153–157.

29. Taylor PE. Delayed postoperative hemorrhage from intracranial aneurysm after craniotomy for tumor. Neurology. 1961; 11:225–231.

30. Andrews BT, Raffel C, Rosegay H. Subarachnoid hemorrhage from a peripheral intracranial aneurysm associated with malignant glioma: report of a case. Neurosurgery. 1985; 17:645–649.

31. Mangiardi JR, Aleksic SN, Lifshitz M, Pinto R, Budzilovic GN, Pearson J. Coincidental pituitary adenoma and cerebral aneurysm with pathological findings. Surg Neurol. 1983; 19:38–41.

32. Roitberg BZ, Cochran EJ, Thornton J, Charbel FT. Giant anterior communicating artery aneurysm infiltrated with a primary cerebral lymphoma: case report. Neurosurgery. 2000; 47:458–462.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download