Abstract
Background
Therapeutic approaches to brain metastases include surgery, whole-brain radiotherapy, stereotactic radiosurgery (SRS), and combination therapy. Recently, postoperative or preoperative SRS draws more attention to reduce postoperative recurrence in brain metastases. The goal of this study is to review surgical outcome of patients who had been treated by SRS, and to discuss the effectiveness of preoperative SRS.
Methods
From 2009 to 2015, 174 patients were treated by SRS for brain metastases, and among these 50 patients underwent surgery. Eighteen patients underwent surgery after SRS, and 14 had oligometastases. The patients' median age at the time of surgery was 56 years (range, 34–84 years). The median follow-up duration was 16.5 months (range, 4–47 months). Pathological findings were classified as follows; radiation necrosis (Group I, n=3), mixed type (Group II, n=2), and tumor-dominant group (Group III, n=9). We compared surgical outcome in respect of steroid, mannitol dosage, Karnofsky performance scale, and pathological subgroups.
Results
The median overall survival was 11 months (range, 2–40 months). Six, 12 and 24 months survival rate was 64.3, 42.9, and 28.6%, respectively. Improvement of Karnofsky performance score was achieved in 50% after surgery. The overall survival of Group I (26.6 months) was longer than the other groups (11.5 months). Additionally the patients were able to be weaned from medications, such as steroid administration after surgery was reduced in 10 cases, and mannitol dosage was reduced in 6 cases. Time interval within 3 months between SRS and surgery seemed to be related with better local control.
Conclusion
Surgical resection after radiologically and symptomatically progressed brain metastases previously treated with SRS seems to be effective in rapid symptom relief and provides an improvement in the quality of life. A short time interval between SRS and surgical resection seems to be associated with good local tumor control.
Due to the advancement in the diagnosis and treatment of systemic cancers, the number of patients living with metastatic brain tumor has been increasing dramatically in recent years. Recently, incidence of patients with brain metastases rose much higher than the primary brain malignant brain tumor in the ratio of 10:1 [1]. Treatment options for metastatic brain tumors include surgical resection, whole-brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), chemotherapy and combination therapy.
The management policy of metastatic brain tumor has been changed over the last several decades. In the late 1960s through the mid-1980s, WBRT for brain metastases was a mainstay [23], and surgery was considered only palliative therapy for patients with single metastases. As the role of surgery has been emphasized for immediate functional improvement, combination treatment of surgery and WBRT became a trend in the 1980s [4]. Since the 1990s, SRS became a common treatment option for patients with metastatic brain tumors due to its advantage over WBRT. More recently, Halasz et al. [5] reported that patients treated for fewer than 4 brain metastases with SRS alone had longer survival than those treated with WBRT. The less-invasiveness and equivalent effectiveness of SRS have made it the treatment of choice in a specific patients group: small sized metastases; small number of metastases; no mass effect; and no neurologic deficit [6].
However, radiation necrosis after SRS is a common occurrence. In many cases, radiation necrosis is asymptomatic and resolves over time. However some cases of radiation necrosis result in progressive neurologic symptoms in spite of corticosteroid pulse therapy. In these situations, the differentiation between tumor progression and radiation necrosis is so challenging given the current imaging methodology that clinical decision making remains a difficult problem.
This study was designed to evaluate the surgical outcomes in patients who underwent palliative resection for increasing mass effect or neurological deterioration after SRS.
A retrospective analysis was done in the patients who underwent surgical resection for metastatic brain tumors previously treated with SRS. One hundred seventy-four patients were treated with SRS for metastatic brain tumors between January 2009 and December 2015. Of these, eighteen patients received surgical resection after SRS for the same lesions. We excluded 3 patients with more than four brain metastases and one patient with leptomeningeal seeding. Therefore, a total of 14 patients were finally enrolled in this investigation with Institutional Review Board approval.
The clinical and radiological data were retrospectively collected, which are summarized in Table 1. The median age at the time of surgical resection was 56 years (range, 34–84 years) with a male to female ratio of 1.33:1. The most common primary origin was lung cancer (n=7) followed by breast cancer (n=3). The median follow-up duration was 16.5 months (range, 4–47 months). Among 24 lesions of all brain metastases, in which 19 were from supratentorium and 5 from infratentorium, 14 were surgically intervened. The brain metastases that undergone surgical resection were located in the supratentorial compartment in 11 patients and the infratentorial compartment in 3 patients, accordingly. The frontal lobe was the most affected site (n=5) followed by the cerebellum and occipital lobe (n=3), parietal lobe (n=2) and temporal lobe (n=1).
All patients underwent single session SRS using the Leksell Gamma Knife, Model 4C (Elekta Instrument AB, Stockholm, Sweden). Before surgical resection, fourteen patients with 24 lesions were treated with SRS to the targets with a median dose of 20 Gy at the 55% isodose line (range, 18–21 Gy). The mean treatment volume was 8.2 cc (range, 2.5–15.6 cc). Median time interval between SRS and palliative surgical resection was 8.5 months (range, 0–18 months). Six patients received the two treatments within a 3-month time interval.
At our institution, patients with metastatic brain tumors usually receive steroid pulse therapy for a week following SRS. Patients with deterioration in neurologic function are given additional cycles of steroid. We analyzed the total dose of steroids used one month before the surgery and one month after, and of mannitol used in the same manner. In all patients, surgical resections were performed for the alleviation of mass effect and consequent neurologic symptoms.
Histopathology was confirmed and reviewed by one pathologist (L.S.D). The pathological findings were classified as "Group I: mainly radiation necrosis" (hyalinized vessels and reactive gliosis in the surrounding area of the necrotic core with or without a very few scattered tumor cells) (Fig. 1), "Group II: mixed type" (co-existence of cluster of viable tumor cells and necrotic core) (Fig. 2), or "Group III: mainly tumor-dominant group: (definite tumor cells in all specimen) (Fig. 3). Three patients were included in Group I, II patients in Group II, and 9 patients in Group III.
Gross total resection was accomplished in all patients, with immediate postoperative imaging confirming complete tumor removal. Preoperative and postoperative follow-up MRI data are collected in 13 patients, and one patient is performed with postoperative follow-up CT due to an aggravated general condition. Postoperative follow-up MRIs were obtained at a median of 3 months (range, 1–4 months) after surgery. Local control failure refers to reappearance of enhancing lesions treated with surgery, and distant control failure is defined as the development of any new enhancing lesion in follow-up MRIs. Tumor control was evaluated based on MRI findings by neuro-radiologists. The volumes of tumor and peritumoral edema (PTE) (cc) were measured using (Medical Image Processing, Analysis and Visualization software; Center for Information Technology, National Institutes of Health, version 7.3.0). Evaluation of performance status was measured by Karnofsky performance score (KPS) in this study. KPS was assessed within 1 month of the pre- and post-operative period.
Statistical analyses were done with Version 21.0 of IBM SPSS (Inc., Chicago, IL, USA). The time interval was defined as the time between SRS and surgical resection, and overall survival was calculated from the date of surgery to death by any cause or follow-up loss. The following variables were analyzed for correlation with clinical and functional outcomes: KPS, PTE volumes, dosage of medications such as steroid and mannitol, and pathological subtypes. Kaplan-Meier curves were used to estimate overall survival and local tumor control with the corresponding p values determined by use of the log-rank test. Additionally, recursive partitioning analysis (RPA) classification as prognostic factors for brain metastases is proposed; Class I: patients with KPS ≥70, less than 65 years of age with controlled primary and no extracranial metastases; Class III: KPS<70; Class II: all others [7]. According to the RPA classification, we evaluated overall survival in this investigation. Statistical significance was accepted for p values of <0.05.
The median overall survival was 11 months (range, 2–40 months). Six, 12 and 24 month survival rates were 64.3, 42.9, and 28.6%, respectively (Fig. 4). Thirteen patients (92.8%) were deceased during the follow-up. There was no operative mortality or major surgical complications. Four patients expired within 3 months after surgery in a relatively short term period. Two patients with primary lung cancer expired due to aggravated pneumonia within 2 months after surgery. One patient with colon cancer that metastasizes to the lung had stable local control after surgery but expired due to pneumonia, and one patient with lung cancer metastatic to the liver expired due to acute cholecystitis. Other patients expired from systemic disease progression.
The median overall survival varies depending on the location of tumor with supratentorium being 17.1 months (range, 3–40 months), while infratentorium was 6 months (range, 2–14 months) (p=0.072). And overall survival according to pathologic subgroup was as followings: 26.6 months in Group I (range, 18–32 months), 21 months in Group II (range, 2–40 months), and 9.33 months in Group III (range, 2–24 months). The survival probability between Group I and combined data from Groups II and III was calculated using the Kaplan-Meier method (Fig. 5). There was no significant difference in overall survival between Group I and Group II, III (p=0.310). Additionally, according to the RPA classification, the median overall survival of 24 months (range, 6–40 months) was observed in 3 patients (RPA Class I). The median overall survival was 10 months (range, 2–32 months) in 11 patients with RPA Class II (p=0.096).
Local and distant control failure occurred in 4 and 8 of the 14 patients, respectively. Of these four patients with local control failure, 2 patients experienced tumor recurrence at 2 months after surgery, one at 4 months, and one at 6 months. Median progression free survival of patients who experienced distant control after SRS and surgery was 8 months (range, 1–30 months). The Kaplan-Meier survival curve indicates that local control failure was associated with a time interval of less than 3 months between SRS and surgery (Fig. 6). All 6 patients who underwent surgery within 3 months of SRS exhibited good local tumor control, but the difference is not significant (p=0.129).
Median KPS checked one month before surgery was 90 (range, 70–90), and postoperative median KPS after a month was 90 (range, 50–100). Overall, 7 patients (50%) experienced an improvement in their KPS, 4 patients (28.5%) remained stable and values for 3 patients (21.5%) declined.
Generally, the progression of neurologic deficit is treated with steroids and mannitol as a non-surgical treatment. Fourteen patients were administered dexamethasone preoperatively with an average of 1.5 cycles (range, 1–4 cycles) during 9.4 days (range, 2–27 days). During the postoperative period, the amount of steroid for 10 patients (71.4%) was reduced on average 0.5 cycles (range, 0–1 cycle). Mannitol was used 7.8 times for 8 patients over 8.5 days on average (range, 4–15 days) preoperatively, and postoperatively 6 patients (75%) received a decreased dosage of mannitol.
Postoperative follow-up MRIs were obtained at a median of 3 months (range, 1–4 months) after surgery. We evaluated T2 weighted MRIs after surgical resection and found PTE volumes in pre- and post-operative images was 69.1 cc (range, 11.6–131.1 cc) and 19.0 cc (range, 1.0–58.2 cc), respectively.
Brain metastases occur in 20–40% of patients with systemic cancer [8]. As the treatments for systemic cancer improve and patients live longer, it is reasonable to assume that the number of cancer patients who develop brain metastases will increase [1]. Patients having more than three brain metastases are generally treated with traditional WBRT. However, WBRT is associated with potential adverse radiation effects. In the acute period, patients could suffer from headache, nausea, vomiting and erythema; while delayed radiation effects include somnolence, fatigue and cognitive impairment in many cases [9]. Improvements in stage work-up and follow-up strategy in cancer patients have increased the fraction of solitary or oligo-metastases to the brain. It is reasonable to think that patients with solitary or a few brain metastases benefit from SRS than WBRT, so long as ready detection of distant failure and salvage SRS are possible. Hence SRS is an increasingly common modality used for patients with oligo-metastases [10].
Unlike WBRT, SRS is designed to deliver a high dose of radiation to a focal target in a single session, while minimizing the dose to normal brain tissue. SRS delivers a concentrated high dosage of radiation at directed at a small target and is associated with high incidence of radiation necrosis in brain metastases. The incidence of radiation necrosis has been reported to occur in as many as 50% of patients with metastatic brain tumor treated with SRS [11]. Distinguishing radiation necrosis from tumor recurrence or progression after SRS is very difficult. There is no conclusive diagnostic tool to distinguish radiation necrosis from tumor progression. Kano et al. [12] reported that T1/T2 mismatch were able to differentiate radiation necrosis from tumor progression. However, this method was not predictive in our patients. In our study, 44.4% of patients in Group III which was composed of patients having pathology of mainly tumor-dominant showed T1/T2 mismatch and 2 of 3 patients in Group I (radiation necrosis only) showed T1/T2 match (Fig. 7). The heterogeneity of pathology and the subjectivity in measuring T1/T2 matching seems to be the cause of this discrepancy. In another sense, this discrepancy is also a sign that distinguishing radiation necrosis from tumor progression is very challenging.
In patients with brain metastasis, newly appeared enhancing areas on routine MRI follow-up after SRS could be the result of radiation necrosis, tumor progression, or a combination of the 2 processes. If the enhancing lesion is small, the lesion remains asymptomatic in most cases and short-term image follow-up could be helpful in differential diagnosis. When the lesion is relatively large and symptomatic, most clinicians begin corticosteroid therapy to relieve the symptoms and mass effect under the presumed diagnosis of radiation necrosis. If corticosteroids are not tolerated and the neurological symptoms are aggravated, then surgical resections are considered. Surgical resection remains an essential therapeutic tool, especially in cases requiring immediate relief from neurological symptoms [13]. Moreover, surgical resection is the most definitive way to distinguish radiation necrosis from tumor progression. The sensitivity and specificity of biopsy is more than 95% [14]. Results of the present study correspond with the results of earlier studies, which reported the efficacy of surgical resection in metastatic brain tumors. In our study, many patients were able to be weaned from corticosteroid or mannitol after surgery. In addition, seven patients (50%) experienced an improvement in their KPS immediately after surgery. Additionally, the patients' pathological subgrouping seems to be associated with overall survival outcome. Our results showed that surgical resection for symptomatic enhancing mass after SRS for metastatic brain tumors is effective in early alleviation of neurologic symptoms and could provide valuable information to guide further care.
Another important finding in our study is that a shorter time interval between SRS and surgical resection could be associated with a lower frequency of local failure. There was no local recurrence in patients who underwent surgical resection within 3 months after SRS in our study. This finding suggests the feasibility of neo-adjuvant SRS. Local failure rate of brain metastasis treated with surgical resection without adjuvant WBRT was unacceptably high. However, due to concerns of adverse radiation effects from WBRT, many neurosurgeons preferred SRS as an alternative to WBRT. Initially, postoperative SRS targeting of the resected cavity had emerged as a treatment paradigm to avoid the WBRT. However, theoretically, postoperative SRS has some limitations: 1) SRS after surgical resection cannot prevent the leptomeningeal seeding triggered by open surgery; 2) brain metastasis often requires surgical resection of large areas, and as a result, high dose radiation to the large cavity could be associated with higher rates of radiation necrosis, and 3) defining the target can be very difficult due to postoperative change. More recently, neo-adjuvant SRS prior to surgical resection has been researched as an alternative tool to postoperative SRS to overcome these limitations. Asher et al. [15] published an article addressing the neo-adjuvant SRS before surgical resection for brain metastases. In their series, overall survival rate was 77.8% and 60.0% at 6 and 12 months and local control rate was 97.8% and 85.6% at 6 and 12 months. Patel et al. [16] reviewed records of patients who underwent either preoperative SRS or postoperative SRS and found a lower incidence of leptomeningeal seeding with preoperative SRS.
Our study has important limitations: 1) A small sample size and heterogeneous study population constrained robust statistical analysis; 2) It is impossible to evaluate the role of surgical resections as a palliative measure, because systemic malignancies were in an uncontrolled state in most patients, and 3) Selection bias was inevitable given the retrospective nature of this study.
When treating patients with metastasis, clinicians should take into account several factors such as control of the systemic malignancy, the expectations of patients and their relatives, and cost-effectiveness. The paucity of published data on these patients makes it difficult to determine the optimal treatment. We anticipate that the present study could provide some valuable guidance to clinicians treating patients with cancer metastases to the brain.
It is our belief that surgical resection after radiologically and symptomatically progressed brain metastases previously treated with SRS is effective in rapid symptom relief and provides an improvement in the quality of life. A short time interval between SRS and surgical resection seems to be associated with good local tumor control. This suggests that neo-adjuvant SRS following surgical resection could be a new paradigm in the management of metastatic brain tumors.
Figures and Tables
Fig. 1
A 65-year-old woman with ovarian carcinoma was treated by surgical resection after SRS. Hematoxylin and eosin staining (left ×100, right ×40) of metastatic brain tumor shows necrotic tissue and hyalinized fibrosis. SRS, stereotactic radiosurgery.
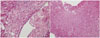
Fig. 2
Hematoxylin and eosin staining in a 48-year-old man with brain metastasis of lung adenocarcinoma shows co-existence of necrotic core (A, ×40) and cluster of viable tumor cells (B, ×200).

Fig. 3
A 34-year-old man presented with confusion, and underwent gross total resection after SRS. A: Hematoxylin and eosin (×100) stain shows highly proliferative malignancy of brain metastasis. B: Tumor cells are positive for thyroid transcription factor-1 (×200). SRS, stereotactic radiosurgery.

Fig. 4
The cumulative overall survival rate in 14 patients who were treated by surgical resection with previous treatment of SRS for brain oligometastases was 64.3, 42.9, and 28.6% at 6, 12, and 24 months, respectively. SRS, stereotactic radiosurgery.

Fig. 5
The survival probability between Group I (radiation necrosis group) and combined Group II (mixed type) and III (tumor recurrence group) was calculated using the Kaplan-Meier method. The overall survival probability of Group I was longer than the other groups, hence, the pathologic subgrouping seems to be associated with the overall survival outcome. However there was no significant difference in overall survival between Group I and Group II, III (p=0.310).

Fig. 6
Kaplan-Meier survival curve demonstrates association of effectiveness between local control and time interval within 3 months of SRS and surgery, which included 6 patients. There was good local tumor control in these patients in our study, however, there was no significant difference in survival (p=0.129). SRS, tereotactic radiosurgery.

Fig. 7
A and B: Axial paired magnetic resonance imaging (MRI) from a patient with ovarian cancer demonstrated a poor correspondence between T1-weighted contrast-enhanced image (A) and the lesion defined by the T2-weighted image (B), indicating a T1/T2 mismatch. Postoperative histopathology shows radiation necrosis. C and D: Axial paired MRI from a patient with breast cancer demonstrated correlation between the margin of contrast enhancement on the T1-weighted image (C) and the clear margin on the T2-weighted image (D), indicating a T1/T2 match. Histopathology reveals radiation necrosis. E and F: Axial paired MRI from a breast cancer revealed a poor correlation of the lesion seen on the T1-weighted image (E) and the lesion on the T2-weighted image (F) (T1/T2 mismatch). Histopathology shows recurrent tumor.
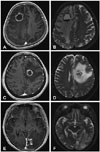
Table 1
Patient characteristics

*The volumes of tumor and peritumoral edema (cc) are measured using Medical Image Processing Analysis and Visualization software, †We reformed the methods introduced in Kano et al. [12] to analyze T1- and T2- MRIs of 14 patients. No., number; OP, operation; PTE, peritumoral edema; SDP, systemic disease progression; SRS, stereotactic radiosurgery. Adapted from Kano H, et al. Neurosurgery 2010;66:486-91, with permission of Neurosurgery [12].
References
1. Stelzer KJ. Epidemiology and prognosis of brain metastases. Surg Neurol Int. 2013; 4:Suppl 4. S192–S202.

2. Order SE, Hellman S, Von Essen CF, Kligerman MM. Improvement in quality of survival following whole-brain irradiation for brain metastasis. Radiology. 1968; 91:149–153.

3. Damiens K, Ayoub JP, Lemieux B, et al. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol. 2012; 19:254–258.

4. Pieper DR, Hess KR, Sawaya RE. Role of surgery in the treatment of brain metastases in patients with breast cancer. Ann Surg Oncol. 1997; 4:481–490.

5. Halasz LM, Uno H, Hughes M, et al. Comparative effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for patients with brain metastases from breast or non-small cell lung cancer. Cancer. 2016; 122:2091–2100.

6. Nieder C, Grosu AL, Gaspar LE. Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol. 2014; 9:155.

7. Videtic GM, Adelstein DJ, Mekhail TM, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for small-cell lung cancer-only brain metastases. Int J Radiat Oncol Biol Phys. 2007; 67:240–243.

9. Baschnagel A, Wolters PL, Camphausen K. Neuropsychological testing and biomarkers in the management of brain metastases. Radiat Oncol. 2008; 3:26.

10. Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994; 28:797–802.

11. Patel U, Patel A, Cobb C, Benkers T, Vermeulen S. The management of brain necrosis as a result of SRS treatment for intra-cranial tumors. Transl Cancer Res. 2014; 3:373–382.
12. Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery. 2010; 66:486–491. discussion 491-2.

14. Lunsford LD. Diagnosis and treatment of mass lesions using the Leksell stereotactic system. In : Lunsford LD, editor. Modern stereotactic neurosurgery. Boston: Martinus Nijhoff Publishing;1988. p. 145–168.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download