Abstract
Background
Although Gamma Knife radiosurgery (GKRS) can provide beneficial therapeutic effects for patients with brain metastases, lesions involving the eloquent areas carry a higher risk of neurologic deterioration after treatment, compared to those located in the non-eloquent areas. We aimed to investigate neurological change of the patients with brain metastases involving the motor cortex (MC) and the relevant factors related to neurological deterioration after GKRS.
Methods
We retrospectively reviewed clinical, radiological and dosimetry data of 51 patients who underwent GKRS for 60 brain metastases involving the MC. Prior to GKRS, motor deficits existed in 26 patients (50.9%). The mean target volume was 3.2 cc (range 0.001–14.1) at the time of GKRS, and the mean prescription dose was 18.6 Gy (range 12–24 Gy).
Results
The actuarial median survival time from GKRS was 19.2±5.0 months. The calculated local tumor control rates at 6 and 12 months after GKRS were 89.7% and 77.4%, respectively. During the median clinical follow-up duration of 12.3±2.6 months (range 1–54 months), 18 patients (35.3%) experienced new or worsened neurologic deficits with a median onset time of 2.5±0.5 months (range 0.3–9.7 months) after GKRS. Among various factors, prescription dose (>20 Gy) was a significant factor for the new or worsened neurologic deficits in univariate (p=0.027) and multivariate (p=0.034) analysis. The managements of 18 patients were steroid medication (n=10), boost radiation therapy (n=5), and surgery (n=3), and neurological improvement was achieved in 9 (50.0%).
Conclusion
In our series, prescription dose (>20 Gy) was significantly related to neurological deterioration after GKRS for brain metastases involving the MC. Therefore, we suggest that careful dose adjustment would be required for lesions involving the MC to avoid neurological deterioration requiring additional treatment in the patients with limited life expectancy.
Metastatic brain tumor, which is known to have a high occurrence of as much as 40% in patients with systemic malignancy, is the most common indicative disease for the Gamma Knife radiosurgery (GKRS), and the local control rate after GKRS is known to be about 70–90% [12345]. With recent advances in treatment for primary malignancies, many cancers have relatively increased life expectancy. These increased survival rate in turn can be expected to result in new, repeated metastatic brain tumor, and taking into consideration the possible adverse events that could result from repeated treatments. Because GKRS is a method that enables repeated treatment of intracranial lesions, it can be a good therapeutic option compared to conventional radiotherapy.
Recently published papers that have reported outcome of GKRS in patients with metastatic brain tumor in eloquent are as noted the occurrence of transient adverse events (neurologic change and seizures) [67]. For metastatic brain tumor patients, not only the therapeutic efficacy but also the preservation of quality of life should be taken importantly in deciding on the right treatment modality, especially when they are not expected to have a long lifespan left. Therefore, physicians must take into consideration the possible neurological complication following radiosurgery on top of the efficacy of the treatment in metastatic brain tumor patients involving eloquent area.
Although GKRS can provide beneficial therapeutic effects for the patients with brain metastases, lesions involving the eloquent areas carry a higher risk of neurologic deterioration after treatment, resulting in poor quality of life of those patients. However, unlike the reports on treatment outcomes, the neurologic deterioration after stereotactic radiosurgery have rarely been reported. Therefore, we aimed to investigate neurological changes of the patients with brain metastases involving the motor cortex (MC) and the relevant factors related to neurological deterioration after GKRS.
We retrospectively reviewed clinical, radiological and dosimetry data of 51 patients who underwent GKRS for 60 brain metastases involving the MC at our institutebetween August 2008 to February 2015. This study was approved by the Institutional Review Board for Human Investigation of Ajou University Hospital, Suwon, Korea (AJIRB-MED-MDB-14-273).
The patients comprised of 26 women and 25 men, and the mean age at the time of GKRS was 58.8 years (range, 35–77 years). The median Karnofsky performance status was 90 (range, 60–100). The primary tumor sites were the lung (n=34), breast (n=8), hepatocellular (n=5), gastrointestinal tract (n=3), and thyroid (n=1). The mean number of brain metastases involving the MC was 1.3 (range, 1–3). There were 25 patients with extracranial metastasis, and 24 patients had their primary tumor being controlled at the time. Whole-brain radiotherapy was given in 14 patients, 7 before GKRS and 7 after GKRS. We defined a "controlled primary tumor" as stable status of primary tumor without new extracranial metastases in the metachronous type, and no extracranial metastases in the synchronous type. When all patients were classified according to recursive partitioning analysis classification, there were 12 (23.5%) class I, 37 (72.6%) class II, and 2 (3.9%) class III patients (Table 1) [8].
Among 51 patients, 23 showed motor weakness and 3 had dysarthria prior to GKRS.
GKRS was performed using a Leksell Gamma Knife (Elekta Instrument, Stockholm, Sweden) model C. The planning system was a Leksell Gamma Plan version 8.3.1 (Elekta Instruments AB). For magnetic resonance imaging (MRI) of radiosurgical planning, T1-weighted axial images with double-dose contrast and T2-weighted axial images were obtained with 2-mm slice thickness without gaps. Mean tumor volume was 3.2 cc (range, 0.001–14.1 cc). GKRS treatment was done with a mean volume coverage of 98.8% (range 96–100%). The mean prescription dose of 18.6 Gy (range, 12–24 Gy) was delivered to the mean 56.3% (range, 50–90) isodose line.
MRI was performed every 3 months, including continuous thin cut T1 enhanced images, the same technique as MRI for GKRS. When tumor progression was suspected on follow-up MRI, perfusion MRI and whole body positron emission tomography-CT were performed to differentiate radiation necrosis and to evaluate systemic disease progression. Tumor volume was calculated as enhancing lesions in T1 enhanced images, and peritumoral edema volume was calculated as T2 abnormal signal volume minus the tumor volume. Volume measurement of tumors and peritumoral edema was performed using the co-registration program (Leksell Gamma Plan®, version 8.3.1 Eleta Instrument AB, Stockholw, Sweden). Local tumor control and peritumoral edema reduction was assessed according to the Macdonald's criteria [910]. Complete response (CR) was defined as complete disappearance of all the lesions, partial response (PR): ≥50% decrease in enhancing tumor volume, progressive disease (PD): ≥25% increase in the lesions, and stable disease (SD): <50% decrease or <25% increase in enhancing tumor volume. We defined local tumor control as "CR, PR, and SD". Neurological deterioration was defined as development of new neurologic deficit or aggravation of pre-existing deficits.
Statistical analysis was performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). To investigate relevant factors, Kaplan-Meier analysis was used for categorical variables. Prognostic factors, including age, gender, number of brain metastases, tumor volume, primary tumor histology, primary tumor control, prescription dose, maximum dose, additional whole brain radiotherapy were assessed using multivariate analysis with Cox proportional hazards model for significant factors. Results were regarded as significant for p<0.05.
The median follow-up duration was 12.3±2.6 months (range, 1–54 months), and the calculated overall median survival time was 19.2±5.0 months after GKRS (Fig. 1A). At the last follow-up, 21 out of 51 patients had died. The cause of death was systemic cancer progression in 19 and unknown in 2 patients. Four patients (7.8%) experienced new brain metastasis and were treated with whole brain radiation therapy.
All 60 brain metastases involving the MC were assessed by at least one imaging follow-up with a mean imaging follow-up duration of 9.8 months (range, 0.6–48.6). Results of local tumor control at the time of the last follow-up were CR in 13 (21.7%), PR in 20 (33.3%), SD in 17 (28.3%), and PD in 10 (16.7%). The calculated local tumor control rates at 6 and 12 months after GKRS were 89.7% and 77.4%, respectively (Fig. 1B).
Among 26 out of 51 patients who showed neurologic deficit prior to GKRS, 12 patients (46.2%) showed improvement in neurologic deterioration, and the median time interval to neurologic deterioration improvement was 0.6±0.1 months (range, 0.2–4.2 months). Eighteen patients (35.3%) experienced new (in 8 patients) or worsened (in 10 patients) neurologic deficits with a median onset time of 2.5±0.5 months (range, 0.3–9.7 months) after GKRS.
Among 18 patients who showed neurologic deterioration, ten patients were suspected as undergoing radiation-induced change and treated with corticosteroid medication. These patients showed improvement of motor function except one patient, and the median time interval to symptom improvement was 0.5±0.6 months (range, 0.1–2.3 months). Eight patients were suspected having tumor progression in follow-up MRI, in those, five patients received boost radiation therapy and three patients underwent surgical resection. However, two patients of them were turned out as radiation necrosis in histopathological examination.
Multivariate analysis was performed to configure factors related to neurologic deterioration, and analysis on prescription dose, new metastases involving the MC, radiation maximum dose, tumor volume, primary tumor site, additional whole brain radiotherapy was done. Prescription dose over 20 Gy was found to have statistical significance (p=0.034) (Table 2, Fig. 2).
Treatment methods for brain metastases continue to develop, and the advantage of GKRS is the relatively simple process and low toxicity compared to conventional surgical resections [711]. There are several treatment plans for metastatic brain tumor, although there had been reports on survival time which showed prolonged survival increment of about 4–12 months in the group who have only have received radiotherapy, compared to the group that has received concurrent operative and radiotherapy [121314]. There also had been reports that this statistically significant survival time difference between the two treatment groups was not seen in metastatic brain tumor patients with less than 4 lesions [13151617].
Many of the metastatic brain tumor patients do not have a long life expectancy that disabilities that may result from post-treatment adverse events may come to them as a much bigger deranging factor of life quality. The chances of complication such as motor weakness are more common in treating lesions involving the eloquent areas. These complications do not only debase the quality of life in patients but also affect the treatment of primary malignancy in a negative way, that the right treatment paradigm to minimize complication is of utmost importance.
Dea et al. [7] published a paper about GKRS treatment for a patient with a brain tumor at the eloquent area. The median time to tumor progression was 16 months. New neurologic deficit occurred in 5.7% and the neurologic deficits were improved soon. Higher margin dose, absence of edema, non-small cell lung cancer tissue type factor were the major factors affecting the good response rate. In the paper, the authors analysis for the therapeutic effect, but they did not analyze for the cause of the neurologic deficit. Their study included all of the eloquent areas such as the primary motor, somatosensory, speech, and visual cortices, resulting in relatively low incidence of motor deterioration.
Luther et al. [18] have reported the outcomes of patients who have undergone stereotactic radiosurgery for metastatic brain tumor involving the MC, which resulted in eventual changes in motor function. Of the 47 patients who have had normal motor function before treatment, ten patients (22%) of previously non-symptomatic patients had newly developed motor weaknesses. Also, those tumors with size over 9 cm3 had statistically significant finding of motor function deterioration. In their study, 19% (18/96) of patients had either developed new neurologic symptoms or deteriorated. 13 patients were treated with corticosteroid medication, 3 with boost radiation therapy, and 2 with surgical resection. Their study included metastatic tumor ≥1.5 cm in greatest dimension with 12–20 Gy prescription dose. By giving relatively low dose, neurologic deterioration was less common than our results in the study. However, local tumor control rate was not presented in that study.
In our study, of the presumed factors affecting neurological deterioration, prescription dose over 20 Gy was the statistically significant factors. Radiation maximum dose, tumor volume, primary tumor site, additional whole brain radiotherapy did not show statistical significance in relation to neurological change in this study. Application of prescription dose over 20 Gy could be good for local tumor control, through this could increase tumor necrosis and/or peritumoral edema, which could result in a neurologic deficit of the patients.
The mean prescription dose used in our study was 18.4 Gy, and the local tumor control rate at 6–month and 12–month point were 87.9% and 83.3%, respectively, not much different from previously reported papers. Sufficient GKRS effect can be seen with prescription dose under 20 Gy, and this could, in turn, mitigate the development of neurological deterioration.
In conclusion, significant factors related to neurological deterioration after GKRS in our series revealed to be prescription dose of over 20 Gy. Therefore, we suggest that careful dose adjustment be implementedfor brain metastases involving the MC to avoid post-surgical neurological deterioration that may require additional treatment after GKRS.
Figures and Tables
 | Fig. 1Kaplan-Meier curves for the patients with brain metastases involving the motor cortex after gamma knife radiosurgery. A: Overall survival. B: Recurrence-free survival. |
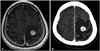 | Fig. 2Illustrative case of a 51-year-old male patient with brain metastases involving the motor cortex from hepatocellular carcinoma. He had no pre-existing neurological deficits. GKRS was performed with a prescription dose of 22 Gy at 50% isodose line. Right hemiparesis occurred two months after GKRS, and treated with intravenous corticosteroid. A: Contrast enhanced T1-weighted image at the time of GKRS. B: Enhanced CT image 1 week after GKRS. GKRS, Gamma Knife radiosurgery. |
Table 1
Patient characteristics

Table 2
Factors related to neurologic deterioration after GKRS

References
1. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003; 52:1318–1326. discussion 1326.

2. Joseph J, Adler JR, Cox RS, Hancock SL. Linear accelerator-based stereotaxic radiosurgery for brain metastases:the influence of number of lesions on survival. J Clin Oncol. 1996; 14:1085–1092.

3. Kondziolka D, Martin JJ, Flickinger JC, et al. Long-term survivors after gamma knife radiosurgery for brain metastases. Cancer. 2005; 104:2784–2791.

4. Kano H, Iyer A, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Outcome predictors of gamma knife radiosurgery for renal cell carcinoma metastases. Neurosurgery. 2011; 69:1232–1239.

5. Auchter RM, Lamond JP, Alexander E, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996; 35:27–35.

6. Davidson L, Zada G, Yu C, et al. Delayed toxicity from gamma knife radiosurgery to lesions in and adjacent to the brainstem. J Clin Neurosci. 2009; 16:1139–1147.

7. Dea N, Borduas M, Kenny B, Fortin D, Mathieu D. Safety and efficacy of Gamma Knife surgery for brain metastases in eloquent locations. J Neurosurg. 2010; 113:Suppl. 79–83.

8. Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000; 47:1001–1006.

9. Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol. 2008; 29:419–424.

10. Molenaar R, Wiggenraad R, Verbeek-de Kanter A, Walchenbach R, Vecht C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg. 2009; 23:170–178.

11. Petrovich Z, Yu C, Giannotta SL, O'Day S, Apuzzo ML. Survival and pattern of failure in brain metastasis treated with stereotactic gamma knife radiosurgery. J Neurosurg. 2002; 97:5 Suppl. 499–506.

12. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322:494–500.

13. Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996; 78:1470–1476.

14. DeAngelis LM, Mandell LR, Thaler HT, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989; 24:798–805.

15. Gerosa M, Nicolato A, Foroni R, et al. Gamma knife radiosurgery for brain metastases: a primary therapeutic option. J Neurosurg. 2002; 97:5 Suppl. 515–524.

16. Gerosa M, Nicolato A, Foroni R. The role of gamma knife radiosurgery in the treatment of primary and metastatic brain tumors. Curr Opin Oncol. 2003; 15:188–196.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download