Abstract
Background
Non-functioning pituitary adenomas (NFPA) are clinically challenging because they present at a late stage with local mass effects or hypopituitarism. Surgery for non-functioning pituitary adenoma requires a special strategic approach for both minimal morbidity and radical resection. However, the clinical predictive factors associated with recurrence are limited. Here, we investigated optimal treatment of non-functioning pituitary adenoma.
Methods
We enrolled 289 patients who presented with non-functioning pituitary adenoma between January 2000 and January 2012 and who had received follow-up for at least one year for this retrospective study. Of these patients, 152 were male and 137 were female, with a median age of 51 years (range 15.79 years) and a median follow-up of four years (range 1.12.6 years). Characteristics of patients and tumors were reviewed with electronic medical records and radiologic images, retrospectively.
Results
Of the tumors, 193 were gross-totally resected, 53 were near-totally resected, and 43 were sub-totally resected. The extent of resection and adjuvant radiotherapy were both statistically significant prognostic factors of recurrence. Immunohistochemistry of tumor specimens did not yield consistent results.
Non-functioning pituitary adenoma (NFPA) is the most frequent type of pituitary tumor. This benign tumor is usually not life threatening and often presents as a macroadenoma larger than 10 mm. These macroadenomas can cause symptoms related to mass effect in which the optic apparatus is compressed, characteristically resulting in a bitemporal hemianopia. Patients can also suffer hypopituitarism secondary to compression of the normal pituitary gland. Most patients require surgery, and a trans-sphenoidal approach is considered the treatment of choice. Radical removal of the tumor mass results in improvement of visual symptoms in 80% of patients and relief of headache in almost 100% of patients [1], with most patients recovering completely after surgery.
A previous meta-analysis [2] found that most recurrence of NFPA occurs between one and five years after surgery, and that the rate of recurrence decreases after 10 years. However, in our experience, a significant proportion of NFPA recurs following a successful initial operation in the long-term follow-up period, suggesting that a longer follow-up period might reveal a higher recurrence rate. Despite possible tumor regrowth, recurrence cannot be detected before it is large enough to cause symptoms. NFPA is not related to the longevity survival and patients can expect normal life expectancy. Therefore, surgical resection of NFPA should not result in significant complications or morbidities. Residual or recurrent tumor can be treated with radiotherapy, stereotactic radiosurgery, or repeat surgery. However, obliteration of surgical landmarks, formation of scar tissue, and the effects of preoperative radiotherapy or medical treatment contribute to the difficulty of a repeat surgery. Complications are frequently followed by repeat surgery, and the rates of clinical remission are lower than those observed following a primary operation [3]. Radiotherapy or radiosurgery can be administered postoperatively as prophylaxis to inhibit recurrent growth.
Recurrence of NFPA after remission is well-recognized. However, the clinical predictive factors associated with recurrence are limited. Thus, the optimal treatment regimen and duration for this tumor have not yet been established. The purpose of this study was to determine the clinical outcome and risk factors of recurrence in order to provide an optimal therapeutic plan for NFPA.
Between January 2000 and January 2012, 1,592 consecutive patients underwent surgery for pituitary tumor at Samsung Medical Center. All surgical procedures were performed by one surgeon (Kim JH). With regard to the endocrine status of these tumors, 1,050 were hormone-secreting, functioning pituitary adenoma and were excluded from this study. From the remaining patients, those with insufficient data (such as loss of medical record, loss of MRI, or follow-up less than one year) and those initially treated at another institution were excluded from this study. The remaining 289 patients were enrolled in the study. Of these, 282 patients (97.6%) underwent surgery via the trans-sphenoidal approach, whereas the remaining seven patients (2.4%) received the trans-cranial approach. All patients underwent general anesthesia. In most cases, the standard microsurgical transseptal approach was used. The anterior wall of the sphenoid sinus was opened bilaterally, and any septum in the sphenoid was removed. The floor of the sella was opened with a high-speed drill, and the tumor was removed with standard instruments of the transsphenoidal approach. After tumor removal, the floor of the sella was reconstructed in most cases with harvested autograft abdominal fat and septal bone.
Initially, all tumors were diagnosed radiologically with MRI. In addition, MRI and CT results were evaluated to determine the size of tumor, extent of tumor, and invasion into the cavernous sinus. The size of the tumor was measured on the coronal view of gadolinium-enhanced T1-weighted image. The longest diameter measured was considered the tumor size. Post-operatively, MRI was performed in all patients to evaluate residual tumor. Gross-total resection (GTR) was defined as no residual enhancing mass on post-operative MRI. Near-total resection (NTR) was defined as suspicious residual enhancing mass on post-operative MRI or residual mass less than 10% of the initial size. Subtotal resection (STR) was defined as a significant residual mass greater than 10% of the initial size. We regarded the regrowth of a remnant tumor as a recurrence, because most recurrent pituitary tumors originate from remnant tumors. All treatments performed for residual masses before evidence of regrowth of residual tumor were considered adjuvant treatment. Conventional radiotherapy (RT) or gamma knife radiosurgery (GKS) was performed for adjuvant treatment of residual enhancing mass. After surgery, all tissue specimens were examined by specialized neuropathologists and were confirmed to be pituitary adenoma. The remnant tissue specimens were examined using immunohistochemistry.
Data for continuous variables are presented as mean or median, while data for categorical variables are presented as number and/or percentage. Time to event data were summarized using Kaplan-Meier plots, and the log-rank test was used to determine statistical differences between groups. Univariable and multivariable analyses were performed using with the Cox regression model. Statistical analyses were carried out using commercial software (SPSS, version 20; IBM, Armonk, NY, USA). Results were considered significant for p values<0.05.
From the database of our institution, 289 patients (152 male, 137 female) with a median age of 51 years (range 15–79 years) and a median follow-up of four years (range 1–12.6 years) were included in the present study. Of these, 198 patients (68.5%) had follow-up longer than three years, 115 patients (39.7%) had follow-up longer than five years, and 23 patients (7.9%) had follow-up longer than 10 years. Most frequently, patients reported mass-related symptoms, i.e., visual symptoms in 149 (51.5%) and headache in 96 (33.2%). Thirty-nine patients (13.4%) were diagnosed incidentally. The median tumor size was 27.8 mm (range: 9–90 mm). Eighty-one tumors (28.0%) extended to the supra-sellar region and reached the third ventricle. Ten tumors (3.5%) extended to the infra-sellar region with invasion of the sellar turcica. Radiologic cavernous sinus invasion was observed in 43 tumors (14.9%) (Table 1).
Most of the cases, i.e., 282 (97.6%), were conducted using a trans-sphenoidal approach, while the remaining seven cases (2.4%) were conducted with a trans-cranial approach. Those cases conducted with a trans-cranial approach had undergone initial surgery before 2007 and all had tumors extended to the supra-sellar region and showed involvement of the cavernous sinus.
During the follow-up periods, 47 cases were recurred. Among them, 10 cases received GKS, 7 cases received RT, 4 cases had to receive a re-operation, and the other 26 cases were observed without any treatment. The progression free survival (PFS) was 83% at five years and 60% at 10 years for the entire cohort of cases. In totally, 193 tumors were gross-totally resected. There was no evidence of residual lesions on post-operative imaging, but 16 tumors recurred during the follow-up period. Fifty-three tumors were near-totally resected. Among them, four had received adjuvant treatment. None of these tumors were recurrent. However, of the other 49 cases that had not received adjuvant treatment, 14 tumors (28.5%) were recurrent. Forty-three tumors were sub-totally resected. Among them, 19 cases received adjuvant treatment, and three of these tumors were recurrent. The other 24 cases had not received adjuvant treatment, and 14 of these tumors (58.3%) were recurrent.
Multivariable analysis was performed to determine the factors that predicted improved outcomes. On multivariable analysis, potential prognostic factors.age, sex, extent of resection, and whether adjuvant treatments were considered. Age, sex were excluded as independent prognostic factors, while the remaining factors.extent of resection, and whether adjuvant treatment were identified as independent prognostic factors related to prolonged PFS (Table 2).
Cases were classified into five groups based on the extent of resection and administration of adjuvant treatment. Group 1 (GTR) had a five-year PFS of 95%. Group 2 (NTR with adjuvant treatment) showed a five-year PFS of 100%. Group 3 (NTR without adjuvant treatment) showed a 65% five-year PFS, which was statistically significantly different from Group 1 (p<0.001). Group 4 (STR with adjuvant treatment) showed an 80% five-year PFS, and Group 5 (STR without adjuvant treatment) showed an only 35% five-year PFS, which was statistically significantly different from Group 1 (p<0.001) (Fig. 1, Table 3).
There were no statistically significant differences in age or sex between cases with or without recurrence. Among cases of recurrence, tumor control rate was measured, excluding those cases for which the follow-up was less than one year after re-treatment. One of the seven cases that underwent RT experienced recurrence. One of the 17 cases that underwent GKS ex-perienced recurrence. Among the 289 patients in this study, 263 had available tumor immunohistochemistry data. Adrenocorticotropic hormone, thyroid-stimulating hormone, follicle stimulating hormone, luteinizing hormone, growth hormone, prolactin (PRL), and Ki-67 were analyzed; however, none of these factors showed statistical significance for recurrence of NFPA (Table 4).
Of the total patients, 142 (50.2%) were prescribed hormonal replacement therapy after the operation. Among them, 36 patients (12.5%) were able to discontinue medication after six months, although 83 patients (28.7%) had to take the medication for longer than one year. Among the 198 patients with follow-up longer than three years, 69 (34.8%) had to take a hormone replacement. There was no difference in frequency of hormone replacement therapy prescribed among the groups based on extent of resection (GTR, NTR, and STR). Considering the effect of radiation therapy, only 3 of 23 patients were prescribed hormone replacement after adjuvant radiation therapy. There were no statistical differences in frequency of hormone replacement prescription before and after radiation therapy.
There was one case of re-operation due to cerebrospinal fluid leakage. Four cases had intracranial hemorrhage on postoperative CT scan (sub-arachnoid hemorrhage, one case; intra-cerebral hemorrhage, three cases). Three cases of intra-cerebral hemorrhage received re-operation without any neu-rologic deficits. There was one case of postoperative intra-cerebral hemorrhage with intra-ventricular hemorrhage, which was a revision operation in a recurred case. The patient progressed to a vegetative state. Postoperative intracranial infection was detected in two cases, one of which lead to death due to severe ventriculitis.
A 22-year-old man visited our outpatient department presenting with visual field defects. Initial MRI studies showed a supra-sellar extending enhancing mass, suggesting pituitary adenoma. Baseline hormonal evaluation of the patient reveal-ed values within reference ranges. The tumor was removed near-totally via the trans-sphenoidal approach. A suspicious residual mass was identified on the upper left side of sella. MRI studies were repeated one year after the operation, and progressive growth of the enhancing nodular lesion was detected. The patient was lost to follow-up without any further treatment. Six years after the operation, the patient visited the outpatient department presenting with lethargy and weight loss. MRI showed a significantly progressed pituitary adenoma with hydrocephalus. The patient underwent surgery again via the trans-sphenoidal approach. The tumor was removed sub-totally. Unfortunately, postoperative intra-cerebral hemorrhage with intra-ventricular hemorrhage resulted in a deteriorated mental status. The patient required full-time care in a bed-ridden state. An adjuvant GKS was conducted to the residual tumor for growth control (Fig. 2).
NFPA has been reported to have a low probability of recur-rence, in part because many patients are not diagnosed until the recurrent tumor is large enough to cause a mass effect (i.e., compression of the optic apparatus or other structures). From this study, the PFS of NFPA was 60% at 10 years. These data suggest that a longer follow-up might reveal eventual tumor recurrence, and that it is important to find strategies to minimize recurrence. However, there is no consensus on the prognostic predictors of NFPA recurrence. Indeed, no single convincing prognostic factor for recurrence of NFPA was identi-fied in a previous meta-analysis study on NFPA [2]. In most studies, clinical factors of age, gender, tumor size and tumor invasion have had no predictive value on recurrence. Recently, many other factors that influence the proliferation of pituitary adenomas, such as angiogenesis, apoptosis, growth factors, oncogenes, tumor suppressor genes and hormone recep-tors, were introduced [456]. Ki-67 has been suggested as an independent marker of tumor progression and recurrence in pituitary adenomas in some previous studies [678]. However, in our study, Ki-67 was inconclusive as a prognostic indicator, possibly due to a lack of sufficient sample size. Still, such immunohistochemical staining approaches could help in determining whether patients need intensive follow-up MRI.
In this study, patients with no residual tumor on postoperative imaging and patients who underwent adjuvant radiotherapy (RT or GKS) showed a similar risk of tumor recurrence. This finding is in agreement with previous reports [91011] and suggests that gross total removal and/or adjuvant radiotherapy prevent the recurrence or regrowth of NFPA.
RT is usually reserved for NFPA patients whose tumors have the potential to behave more aggressively, are of considerable size, or have recurrence that might not be amenable to further surgery. In NFPA patients, RT results in excellent long-term local tumor control with reported rates of 90–97% at 10 years [12131415]. Previously, we found RT and GKS were efficient treatment modalities for the control of tumor growth in patients with pituitary adenomas; PFS was 99% at 2 years and 97% at 4 years, with no significant differences between the RT group and the GKS group [16]. Another study found that there was no major influence of RT on cognition in NFPA patients treated with RT compared to those treated with surgery alone [1718]. Brain abnormalities on MRI (white matter lesions and cerebral atrophy) were not observed more frequently in NFPA patients treated with postoperative RT compared to patients treated with surgery-alone [19]. Adjuvant radiotherapy should not be delayed because of such marginal complication rates. Radiosurgery such as GKS has reported fewer complications than RT; hence, it can be a better option for adjuvant treatment. We would like to recommend receiving GKS for adjuvant radiotherapy, when the residual mass is detected.
Considering the complications of radiation, gross total removal should be the goal of surgery. However, it is generally recognized that radical surgery is not possible in some patients with pituitary adenomas, and approximately 15–20% of patients require repeat surgery because of tumor recurrence [2021]. A meta-analysis of previous studies has found that the remission percentage of transsphenoidal surgery has not improved over the last three decades [2]. The majority of papers in that study were based on traditional surgery using microscopy. Endoscopic techniques have recently improved and are still advancing. The endoscopic technique is associated with shorter hospital stay, less blood loss, fewer nasal complications, and less frequent diabetes insipidus than conventional microscopic technique [222324]. One of the advantages of using an endoscope is the better view and resection of residual tumors due to the possibility of introducing the endoscope into the sella turcica and supra-sellar region, which would facilitate surgical cure [25]. Moreover, this might prevent major bleeding in the cavernous sinus and lesions in the internal carotid artery [26]. This endoscopic surgery might lead to better results in cases of NFPA.
In conclusion, NFPA is not a life threatening disease, and the patients can expect full life expectancy. There should not be significant complications or morbidities after operation. However, based on our experience and the findings of this study, there is a significant recurrence rate of NFPA even in long-term follow-up. Recurrence is one of the most troublesome clinical outcomes of NFPA. To reduce the recurrence rate of NFPA, neurosurgeons must attempt GTR. In addition, talented skill or endoscopic technique can be helpful. Despite all efforts for a cure, residual tumor can be controlled by adjuvant radiotherapy, especially GKS, with a minimal complication rate. We would like to recommend receiving GKS for adjuvant radiotherapy, when the residual mass is detected. Even though immunohistochemistry showed no promising results in the current study, a larger study population size will be needed to validate this finding. Investigation of the genetic pathophysiology of NFPA and improvement of surgical technique with advanced surgical equipment will lead to improved patient outcome.
Figures and Tables
Fig. 1
Progression-free survival between groups based on extent of resection and administration of adjuvant treatment. GTR, gross total removal; NTR, near total removal; STR, subtotal removal; Tx, treatment.

Fig. 2
Illustrative case. A 22-year-old male patient presented with visual field defects. A: Initial MRI showed pituitary adenoma. B: Suspicious residual mass identified after operation. C: 1-yr postoperative MRI showed progression. The patient was lost to follow-up without any further treatment. D: 6-yr postoperative MRI showed significantly progression with hydrocephalus. E: After the second operation, intra-cerebral hemorrhage with intra-ventricular hemorrhage occurred. F: An adjuvant gamma knife radiosurgery was conducted to the residual tumor.
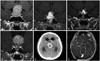
Table 1
Clinical characteristics of patients and tumor with nonfunctioning pituitary adenomas

Table 2
Survival analysis for progression free survival

Table 3
Comparing progression-free survival between the groups based on the extent of resection and administration of adjuvant treatment

Acknowledgments
This article is dedicated to Professor Kim Jong Hyun, who inspired us to become creative, active, and positive neurosurgeons.
References
1. Mortini P, Barzaghi R, Losa M, Boari N, Giovanelli M. Surgical treatment of giant pituitary adenomas: strategies and results in a series of 95 consecutive patients. Neurosurgery. 2007; 60:993–1002. discussion 1003-4.
2. Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. 2012; 15:71–83.

3. Laws ER Jr, Fode NC, Redmond MJ. Transsphenoidal surgery following unsuccessful prior therapy. An assessment of benefits and risks in 158 patients. J Neurosurg. 1985; 63:823–829.
4. Farrell WE, Clayton RN. Molecular pathogenesis of pituitary tumors. Front Neuroendocrinol. 2000; 21:174–198.

5. Saeger W. Pituitary tumors: prognostic indicators. Endocrine. 2005; 28:57–66.
6. Noh TW, Jeong HJ, Lee MK, Kim TS, Kim SH, Lee EJ. Predicting recurrence of nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2009; 94:4406–4413.

7. Gejman R, Swearingen B, Hedley-Whyte ET. Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol. 2008; 39:758–766.

8. Šteňo A, Bocko J, Rychlý B, et al. Nonfunctioning pituitary adenomas: association of Ki-67 and HMGA-1 labeling indices with residual tumor growth. Acta Neurochir (Wien). 2014; 156:451–461. discussion 461.

9. Ferrante E, Ferraroni M, Castrignanò T, et al. Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. Eur J Endocrinol. 2006; 155:823–829.

10. Boelaert K, Gittoes NJ. Radiotherapy for non-functioning pituitary adenomas. Eur J Endocrinol. 2001; 144:569–575.

11. Greenman Y, Ouaknine G, Veshchev I, Reider-Groswasser II, Segev Y, Stern N. Postoperative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumour quiescence and regrowth. Clin Endocrinol (Oxf). 2003; 58:763–769.

12. Park P, Chandler WF, Barkan AL, et al. The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery. 2004; 55:100–106. discussion 106-7.

13. van den Bergh AC, van den Berg G, Schoorl MA, et al. Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int J Radiat Oncol Biol Phys. 2007; 67:863–869.

14. Erridge SC, Conkey DS, Stockton D, et al. Radiotherapy for pituitary adenomas: long-term efficacy and toxicity. Radiother Oncol. 2009; 93:597–601.

15. Gittoes NJ, Bates AS, Tse W, et al. Radiotherapy for non-function pituitary tumours. Clin Endocrinol (Oxf). 1998; 48:331–337.
16. Kong DS, Lee JI, Lim DH, et al. The efficacy of fractionated radiotherapy and stereotactic radiosurgery for pituitary adenomas: long-term results of 125 consecutive patients treated in a single institution. Cancer. 2007; 110:854–860.

17. van Beek AP, van den Bergh AC, van den Berg LM, et al. Radiotherapy is not associated with reduced quality of life and cognitive function in patients treated for nonfunctioning pituitary adenoma. Int J Radiat Oncol Biol Phys. 2007; 68:986–991.

18. Brummelman P, Elderson MF, Dullaart RP, et al. Cognitive functioning in patients treated for nonfunctioning pituitary macroadenoma and the effects of pituitary radiotherapy. Clin Endocrinol (Oxf). 2011; 74:481–487.

19. Sattler MG, Meiners LC, Sluiter WJ, et al. Brain abnormalities on MRI in non-functioning pituitary adenoma patients treated with or without postoperative radiotherapy. Radiother Oncol. 2015; 114:239–244.

20. Auer LM, Clarici G. The first 100 transsphenoidally operated pituitary adenomas in a non-specialised centre: surgical results and tumour-recurrence. Neurol Res. 1985; 7:153–160.

21. Ramm-Pettersen J, Berg-Johnsen J, Hol PK, et al. Intra-operative MRI facilitates tumour resection during trans-sphenoidal surgery for pituitary adenomas. Acta Neurochir (Wien). 2011; 153:1367–1373.

22. Goudakos JK, Markou KD, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery: a systematic review and meta-analysis. Clin Otolaryngol. 2011; 36:212–220.

23. Strychowsky J, Nayan S, Reddy K, Farrokhyar F, Sommer D. Purely endoscopic transsphenoidal surgery versus traditional microsurgery for resection of pituitary adenomas: systematic review. J Otolaryngol Head Neck Surg. 2011; 40:175–185.
24. Rotenberg B, Tam S, Ryu WH, Duggal N. Microscopic versus endoscopic pituitary surgery: a systematic review. Laryngoscope. 2010; 120:1292–1297.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download