Abstract
Background
Recent studies suggest aggressive management combining a grossly total resection (GTR) with adjuvant radiotherapy (RT) as a treatment of choice for intracranial hemangiopericytoma (HPC). However, in these papers, the definitions of complete or GTR are equivocal. In the present study, we reviewed the relevant cases from our experience focused on the clinical efficacy of surgical grading of resection, and analyzed the optimal treatment strategies as well.
Methods
From January 1995 through December 2014, 17 patients treated for intracranial HPC were included in this study. We analyzed clinical presentation, radiologic appearance, pathologic diagnosis, extent of resection, and follow-up outcomes.
Results
A total of 26 operations were performed including 9 recurrent intracranial HPCs. Every tumor was single and had no evidence of metastasis. Most common area of tumor was parasagittal (8 patients, 47.1%), which is adjoined to superior sagittal sinus. For the initial operation, GTR was performed in 16 cases (61.5%), partial resection (PR) in 8 cases (30.8%), and an endoscopic biopsy in 2 patients (7.7%). In Simpson grading system, grade 1 was done in 2 patients (7.7%), grade 2 in 11 patients (42.3%) and grade 3 in 3 patients (11.5%). Postoperative RT was delivered in 16 patients (94.1%) regardless of the extent of resection. The median 57.57 Gy (range, 50-60 Gy) was delivered in median 33 fractions (range, 30-40). The extent of resection (conventional classification and Simpson grading system) and adjuvant RT were significantly associated with recurrence-free survival.
Central nervous system (CNS) hemangiopericytoma (HPC) is a rare mesenchymal tumor which was first described by Stout and Murray [1] in 1942. HPC accounts for 2.5% of all meningeal tumors and <1% of all CNS tumors [234]. Since HPC is rare, prior reports about it are largely institutional retrospective studies, therefore, information about management of the disease is scarce. Recent studies insist that the extent of surgical resection and postoperative radiotherapy (RT) correlate with improved overall survival (OS) and recurrence-free survival (RFS). However, the definitions of complete resection or grossly total resection (GTR) in previous studies are equivocal [5678]. In most reported cases, since HPC is attached to the venous sinuses or major vessels, it is actually impossible to literally completely remove the tumor. Assuming that these ambiguous classifications on the extent of resection might have had a measurement bias, we reviewed the cases of 17 our patients with intracranial HPCs focusing on the clinical efficacy of surgical resection graded by Simpson grading system [9] (which is largely used in meningioma removal). The disease features, treatment strategies, and clinical courses of relevant cases from our experience were also analyzed.
We retrospectively reviewed the medical records of the patients with intracranial HPC treated in our institute from January 1995 to December 2014 (i.e., the 20-year period). The total number of patients included was 17. The medical records of those patients were reviewed and analyzed by the authors. The following information was extracted: age, gender, radiologic finding (location, size, existence of adjacent vessels), pathology [World Health Organization (WHO) histopathological grade, Ki-67, mitosis], the extent of resection, radiation (total dose), Karnofsky performance scale (KPS) (preoperative and final score), length of follow-up, recurrence, metastases, and death. Radiologic findings, such as CT and enhanced MR images, were reviewed by experienced neuroradiologists. Operative records, including notes and video, were reviewed to determine gross tumor extension to adjacent vessels and completeness of resection. As in other papers, the extent of resection was divided into three categories (gross total resection, partial resection, biopsy); it was also divided into five categories by Simpson grading system (Table 1) [9]. The pathologic diagnoses and microscopic measurement were performed by experienced neuropathologist. Immunohistochemistry was done in all cases including Ki-67 proliferative index.
Approval for this study was granted by the Institutional Review Board of Seoul St. Mary's Hospital (approval numbers: KIRB 00513-1-002). Statistical analyses were performed using SPSS, version 17.0 (SPSS Inc., Chicago, IL, USA). The OS and RFS were calculated by the Kaplan-Meier analysis. The univariate analysis was conducted using log-rank test. p-value below 0.05 was considered statistically significant.
Patient demographic data are summarized in Table 2. Between January 1995 and December 2014, for the 20-year period, a total of 17 patients underwent microsurgical resection for primary intracranial HPCs at least once. Additionally, a total number of 26 operations were performed for the resection of either primary or recurrent intracranial HPCs. The patient group included 14 (82.4%) males and 3 (17.6%) females. The median age at the time of the initial diagnosis was 48 years (range, 26-73). The most common presenting symptom was headache, followed by vomiting, visual field defects, and seizures.
The preferential area of tumor was parasagittal (8 patients, 47.1%), which is adjoined to superior sagittal sinus. Distributions of the other tumor locations were as follows: tentorial (3 patients, 17.6%), falx (2 patients, 11.8%), convexity (2 patients, 11.8%), cerebellopontine angle (1 patient, 5.9%), and sphenoid wing (1 patient, 5.9%). To evaluate the correlation between location and the extent of resection, these locations were grouped into 2 categories (Table 2): those that adjoin adjacent vessels such as superior sagittal sinus, transverse sinus (Fig. 1), or sigmoid sinus, and those that have no adjacent major vessels (Fig. 2). The results of Fisher's exact test suggest, that the extent of resection was statistically significantly associated neither with the location of tumor, nor with the existence of adjacent vessels (p=0.35 and 0.60, respectively).
Histopathological results of all specimens obtained in each surgery including recurrence were reviewed. 11 tumor specimens (42.3%) were confirmed WHO grade II HPCs and 15 tumor specimens (57.7%) were confirmed WHO grade III HPCs. Of total 17 patients, progression from low grade (WHO grade II) to anaplastic (WHO grade III) HPC was observed in 2 patients among 3 recurred patients (11.8%). The median Ki-67 proliferative index was 14%, with the range from 1% to 40%. In WHO grade II HPCs, the median Ki-67 proliferative index was 4.8%; in WHO grade III HPCs, it was 21.2%.
Treatment modalities consisted of surgery and adjuvant RT. All patients were initially treated with surgery and there were no perioperative mortalities and no unexpected events during the operations. At the initial operation, a GTR was accomplished in 16 cases (61.5%), a partial resection (PR) in 8 cases (30.8%), and an endoscopic biopsy in 2 cases (7.7%). By Simpson grading system (Table 1), GTR was divided into 3 grades, grades 1 to 3. PR was the same as Simpson grade 4 and biopsy was same as Simpson grade 5. Dividing GTR group into Simpson grading system, grade 1 was done in 2 cases (7.7%), grade 2 in 11 cases (42.3%), and grade 3 in 3 patients (11.5%). In most cases, because of the location of tumor adjoined to the venous sinus, surgical resection was possible only by Simpson grade 2 or 3. Venous sinus was preserved in all patients. The location of tumor in 2 patients who underwent surgical resection by Simpson grade 1 was both at convexity. Surgical grading for recurred HPCs was also evaluated (Table 2).
Postoperative RT was delivered in 16 patients (94.1%), regardless of the extent of resection. One patient (case number 9 in Table 2) rejected active treatment after initial surgery. The median 57.57 Gy (range, 50-60 Gy) was delivered in median 33 fractions (range, 30-40). 2 patients (11.8%) underwent stereotactic radiosurgery using cyberknife for the treatment of recurred HPC (total dose of 15 Gy and 27 Gy each, either by 3 fractions).
The clinical outcomes were evaluated using the KPS. The median KPS at final outcome was 65 (initial median KPS was 65.8). The median follow-up period was 55 months, with the range from 10 to 197 months. 2 of the 17 patients died during the follow-up period, and the median OS was 13 months with median OS not reached (Fig. 3). 5 patients (29.4%) developed local recurrence and the median RFS was 51 month (Fig. 4).
Four types of subgroup analysis were performed for the following predictive factors with RFS: the extent of resection (conventional classification and Simpson grading), RT, and pathologic diagnosis. The median time for local recurrence of patient who underwent GTR was 68.3 months, PR was 37.1 months, and biopsy was 3.5 months (in this case, regrowth), which was statistically significant (p=0.003 by log-rank test) (Fig. 5). Additionally, the resection extent grades of intracranial HPC using Simpson grading system was significantly associated with higher RFS (p=0.011 by log-rank test) (Fig. 6). Regarding adjuvant RT, the median time for local recurrence was 80.5 months in patients who received RT against 19.5 months in those who did not (p=0.0003 by log-rank test) (Fig. 7). Although the median time values for local recurrence were 66.2 months and 38.1 months in the patients with WHO grade II HPC and WHO grade III HPC, respectively, the pathological diagnosis of WHO grades II and III was not statistically significantly associated with the time for local recurrence (p=0.49 by log-rank test). Clinical follow-up and additional image studies, such as positron emission tomography-CT scans, did not suggest distant metastases.
HPC arises from Zimmerman pericytes around the endothelial lining of capillaries and postcapillary venules. Therefore, these tumors can occur anywhere where capillaries are found [110]. However, in many reported cases, as well as in our experience, most of intracranial HPCs are located at parasagittal area or at tentorium which is adjoined to the dural sinus [2356711121314151617]. Therefore, unless the dural sinus is sacrificed or reconstructed, it is difficult to literally completely remove the tumor in most cases.
The results of recent studies on the treatment strategies for intracranial HPCs suggest that both the extent of surgical resection and adjuvant RT are associated with significantly improved OS and RFS [256781112131416]. However, as mentioned above, most previous studies do not define the meaning of "complete resection" or "GTR". Only 2 reports define the complete tumor resection as Simpson grade 1 [1819]. The "gross total" or "complete" resection rate reported in the literature varied between 50 and 83% [23468111215172021]. However, because of the location of tumor, these high rates of complete resection appear to be unreliable. The possibility stands that these might not be Simpson grade 1 removal. Similarly, our experience suggests that GTR was accomplished in 64.7%, while Simpson grade 1 removal was achieved in only 11.8% in which tumors were located at convexity.
As suggested by Bassiouni et al. [18], the classification introduced by Simpson [9] in 1957 to describe the resection rate in meningiomas can also be usefully applied in intracranial HPCs, since, in most cases, these tumors are dura-based. We divided GTR into 3 Simpson grades, grades 1 to 3. The results of the statistical analysis of the correlation between the extent of resection and recurrence suggest statistical significance like other several studies (Fig. 6) [1819]. Conventional classification of dividing into GTR, PR and biopsy showed that each class of resection is significantly associated with RFS; Simpson grading system showed statistically significant correlations between each grade and RFS as well. To prevent local recurrence, it seems to be safer to remove the tumor as much as possible, even when the remaining tumor is adjoined to the adjacent structures, such as the dural sinus.
In many series, intracranial HPCs have been shown to recur even after complete GTR. Therefore, several authors have recommended adjuvant RT for a better outcome [25681113141516172021]. Similarly to other series, our experience shows that the patients who received adjuvant RT tend to have longer RFS (Fig. 7). We performed adjuvant RT regardless of the extent of resection and pathologic grade in most of the patients. 16 out of 17 patients (94.1%) received adjuvant RT to the median dose of 57.57 Gy (range, 50 to 60 Gy) by median 33 fractions (range, 30-40 fractions); no significant radiation-related complications were observed. Although our result has serious limitation to compare with other series; a 1 versus 16 match-up among adjuvant RT effectiveness. Additional study should be done later on to certify effectiveness.
The results of the statistical analysis of RFS depending on the pathologic diagnosis suggest that the median time values for local recurrence in the patients with WHO grade II HPC and WHO grade III HPC were 66.2 months and 38.1 months, respectively. However, possibly due to the small number of the investigated cases, this difference did not reach statistical significance.
In conclusion, surgical resection of intracranial HPC, in an attempt to reach Simpson grade 1 removal of the tumor, is necessary to reduce recurrence. But this attempt to reach Simpson grade 1 removal should be performed within the safe limit. Additionally, as recommended before, adjuvant RT should be done to prevent recurrence, even in the patients who have tumor completely resected and who have been diagnosed as WHO grade II HPC.
Figures and Tables
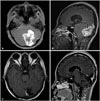 | Fig. 1Representative images of intracranial hemangiopericytoma which adjoins adjacent vessels (case number 17 in Table 2). Magnetic resonance images show heterogenously enhancing mass located in the left tentorium. A: Axial image shows an irregular shaped mushroom-like mass. B: In sagittal image, the mass is bulging bilaterally towards the occipital lobe upwards and cerebellum downwards. C and D: Postoperative axial (C) and sagittal (D) images show the tumor removed gross totally. |
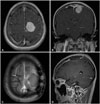 | Fig. 2Representative images of intracranial hemangiopericytoma which has no major vessels adjoined (case number 14 in Table 2). Magnetic resonance images show dural-based enhancing mass located in the left convexity. A and B: Axial (A) and coronal (B) images show a round-shaped homogenously enhancing mass in the left convexity. C and D: Postoperative axial (C) and sagittal (D) images show the tumor removed grossly totally. |
 | Fig. 5Correlation analysis between the extent of resection and recurrence-free survival (log-rank test). GTR, grossly total resection; PR, partial resection. |
 | Fig. 6Correlation analysis between Simpson grading of surgical resection and recurrence-free survival (log-rank test). |
 | Fig. 7Correlation analysis between adjuvant radiotherapy and recurrence-free survival (log-rank test). |
Table 1
Simpson grading system for removal of meningioma [9]

Table 2
Demographic characteristics of 17 intracranial hemangiopericytoma patients

References
1. Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring zimmermann's pericytes. Ann Surg. 1942; 116:26–33.

2. Dufour H, Métellus P, Fuentes S, et al. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery. 2001; 48:756–762. discussion 762-3

3. Goellner JR, Laws ER Jr, Soule EH, Okazaki H. Hemangiopericytoma of the meninges. Mayo Clinic experience. Am J Clin Pathol. 1978; 70:375–380.

4. Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989; 25:514–522.

5. Park BJ, Kim YI, Hong YK, Jeun SS, Lee KS, Lee YS. Clinical analysis of intracranial hemangiopericytoma. J Korean Neurosurg Soc. 2013; 54:309–316.

6. Ghose A, Guha G, Kundu R, Tew J, Chaudhary R. CNS Hemangiopericytoma: A Systematic Review of 523 Patients. Am J Clin Oncol. 2014; 10. 27. [Epub]. DOI: 10.1097/COC.0000000000000146.
7. Tian R, Hao S, Hou Z, et al. Clinical characteristics and prognostic analysis of recurrent hemangiopericytoma in the central nervous system: a review of 46 cases. J Neurooncol. 2013; 115:53–59.

8. Stessin AM, Sison C, Nieto J, Raifu M, Li B. The role of postoperative radiation therapy in the treatment of meningeal hemangiopericytoma-experience from the SEER database. Int J Radiat Oncol Biol Phys. 2013; 85:784–790.

9. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957; 20:22–39.

10. Horten BC, Urich H, Rubinstein LJ, Montague SR. The angioblastic meningioma: a reappraisal of the nosological problem. Light-, electron-microscopic, tissue, and organ culture observations. J Neurol Sci. 1977; 31:387–410.
11. Rutkowski MJ, Jian BJ, Bloch O, et al. Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012; 118:1628–1636.
12. Kumar N, Kumar R, Kapoor R, et al. Intracranial meningeal hemangiopericytoma: 10 years experience of a tertiary care Institute. Acta Neurochir (Wien). 2012; 154:1647–1651.

13. Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma: long-term outcome revisited. Clinical article. J Neurosurg. 2011; 114:747–755.
14. Rutkowski MJ, Sughrue ME, Kane AJ, et al. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. 2010; 113:333–339.

15. Combs SE, Thilmann C, Debus J, Schulz-Ertner D. Precision radiotherapy for hemangiopericytomas of the central nervous system. Cancer. 2005; 104:2457–2465.

16. Kim JH, Jung HW, Kim YS, et al. Meningeal hemangiopericytomas: long-term outcome and biological behavior. Surg Neurol. 2003; 59:47–53. discussion 53-4
17. Bastin KT, Mehta MP. Meningeal hemangiopericytoma: defining the role for radiation therapy. J Neurooncol. 1992; 14:277–287.

18. Bassiouni H, Asgari S, Hübschen U, König HJ, Stolke D. Intracranial hemangiopericytoma: treatment outcomes in a consecutive series. Zentralbl Neurochir. 2007; 68:111–118.

19. Fountas KN, Kapsalaki E, Kassam M, et al. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev. 2006; 29:145–153.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download