Abstract
A 9-month-old male child was brought with complaints of increasing head size for 2 months, increasing lethargy and vomiting for the last 2 days. Radiology revealed a heterogeneously enhancing, globular lesion in the pineal region with hydrocephalus. Near total excision of the tumor was carried out. The histopathological examination of the lesion showed heterogenous elements in the form of mature neuroepithelial and ectomesenchymal tissue. The pathology and radiology of this unusual lesion is discussed with relevant review of literature.
"Pineal parenchymal neoplasms" arise from native neuroepithelial cells, the pineocytes. These comprise of pineocytomas, pineal parenchymal tumors of intermediate differentiation, pineoblastomas, and pineal anlage tumors. The World Health Organization (WHO) in 2007 describes pineal anlage tumors as rare pineoblastomas with melanin production and cartilaginous and/or rhabdomyoblastic differentiation. Seven such cases have been described [1,2]. We report an unusual case of 9 months male child with a pineal tumor showing features that are reminiscent of a pineal anlage tumor however it contained only mature neuroepithelial and ectomesenchymal components and no primitive neuroepithelium. Such a tumor has been reported only once in literature and should be given a name distinct from the anlage tumor which it resembles in part [1,3].
A 9-month-old male child was brought by his parents with complaints of increasing head size for 2 months, increasing lethargy and vomiting for the last 2 days. On examination, the child was irritable and moving limbs spontaneously. His anterior fontanel was tense and bulging. Computed tomography of brain showed a well-defined, heterogeneously enhancing lesion in the pineal region (Fig. 1A, B). Magnetic resonance (MR) imaging revealed 6.1×6.2×7.2 cm lesion with epicenter in the pineal region. It showed mixed intensity on T1 (Fig. 1C), T2 (Fig. 1D) weighted sequences with peripheral cystic areas. The lesion was heterogeneously enhancing (Fig. 1E) and causing triventricular hydrocephalus. Diffusion weighted MR image revealed restricted diffusion suggestive of high cellularity (Fig. 1F). Gradient recovery echo sequence of MR showed multiple dark signal intensities in the tumor suggestive of calcification and absence of blooming effect of hemorrhage (Fig. 1G). The patient was subjected to right ventriculo-peritoneal shunt followed by excision of tumor in the second stage. The patient underwent surgery by the supracerebellar infratentorial approach. The tumor was firm, gritty and vascular. Near total excision of the tumor was done, in which posterior and central part of tumor was excised and shell of tumor anterolaterally left behind. The patient had a stormy postoperative course and succumbed due to septicemia.
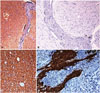
The specimen of tumor showed multiple firm, white glistening tissue bits aggregating to 8 cm and with punctate brown black pigmentation on the cut surface (Fig. 1H). The histopathological examination showed a nodular architecture with nodlarge, focally crowded, and occasional binucleate cells were seen. Hyaline cartilage was seen focally (Fig. 2A-E). The tumor was sampled in toto and did not reveal any primitive neuroepithelial areas or rosettes in neither the surgical specimen nor post mortem specimen. Post mortem examination of the brain revealed a shell of residual tumor anterolaterally without clots in the pineal region (Fig. 2F). Immunohistochemistry showed that the glial cells in the nodules were positive for glial fibrillary acidic protein; the large ganglion cells were positive for synaptophysin but negative for CD34. The meningothelial like cells were positive for vimentin but negative for epithelial membrane antigen. MiB1 labelling index was <1% throughout the tumor (Fig. 3).
Pineal region tumors (PRTs) are a heterogeneous group of tumors accounting for approximately 0.4-1.0% of intracranial tumors. These can be classified into four main categories: germ cell tumors, pineal parenchymal tumors (PPTs), glial cell tumors, and other miscellaneous tumors and cysts. PPTs are even rarer and only approximately 30% of PRTs are PPTs. According to the WHO classification of tumors in the central nervous system, which was revised in 2007, PPTs are subdivided into well differentiated pineocytoma, PPT with intermediate differentiation and pineoblastomas [2,4]. Pineal anlage tumors are added to this list and are described as variants of pineoblastomas with melanin, cartilaginous, and rhabdomyoblastic differentiation [1].
Seven cases of pineal anlage tumors have been documented in the English literature after the original description by Schmidbauer et al. [1,5,6]. A pineal anlage tumor is a primary pineal parenchymal tumor with both neuroepithelial and ectomesenchymal (heterologous) differentiation but without endodermal elements. The neuroepithelial differentiation can include neuronal, glial, and retinal elements. Pigment may be present. Ectomesenchymal differentiation typically includes striated muscle, rhabdomyoblasts, and cartilage formation. The neuroepithelial component of these anlage tumors also contains immature elements including Homer Wright and/or Flexner Wintersteiner rosettes which resembles pineoblastomas [1,6]. Therefore these have been classified as a variant of pineoblastomas and are regarded as WHO grade IV tumors which show an aggressive clinical course and poor prognosis.
The pathological differentials for anlage tumor include melanotic neuroectodermal tumors of infancy and ectomesenchymomas, both of which do contain primitive cells, which were absent in this case. Teratomas and medulloblastomas with myogenic differentiation and/or melanotic differentiation should also be distinguished from pineal anlage tumors. The endodermal (e.g., enteric, respiratory) or cutaneous components typical of teratomas are lacking in pineal anlage tumors. The medulloblastoma variants with divergent differentiation, i.e., medulloblastoma with myogenic differentiation and/or melanotic differentiation must have cerebellar parenchymal involvement by neuroradiologic or intra-operative observation. In equivocal cases, ancillary molecular testing to evaluate for cytogenetic abnormalities suggestive of medulloblastomas (isochromosome 17q, MYC amplification) may be useful in establishing the pathologic diagnosis [1,6].
Radiologically, the tumor in our patient resembled an anlage tumor with its large size and heterogenous enhancement [1]. Pathologically, it contained neuroepithelial components (in the form of glial cells and ganglion cells) and ectomesenchymal components (in the form of hyaline cartilage) similar to an anlage tumor. However; our case was different from all previously reported cases of anlage tumors as all of its components were mature. Only one similar case has been reported in literature, by Gudinaviciene et al. [3] in 2005. Gudinaviciene et al. [3] labelled that tumor a 'pineal anlage tumor without immature elements' and proposed alternative names such as a benign pigmented ectomesenchymoma or an ectomesenchymal hamartoma. Therefore; our case can be regarded as a pineal anlage tumor without immature elements.
However the term pineal anlage tumor connotes a grade IV tumor with a poor prognosis and need of adjuvant therapy, and hence this name may be misleading and inappropriate. The presence of areas resembling meningioangiomatosis is unique to the present case. We believe that this lesion probably represents a dysembryogenic tumor/hamartoma in the pineal region. The long term prognosis remains to be determined.
In conclusion, heterogenous pineal tumors containing mature neuroepithelial and ectomesenchymal components are very rare. These should be considered separate from the anlage tumor and included it in the pathologic differential diagnosis of PRTs.
Figures and Tables
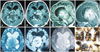 | Fig. 1Radiology of the tumor. A: Plain computed tomography of brain showing a well-defined, heterogenous lesion in the pineal region. B: Post contrast computed tomography showing patchy heterogenous enhancement of the lesion. Magnetic resonance (MR) imaging showed mixed intensity on T1 (C), T2 (D) weighted sequences with peripheral cystic areas and heterogeneous enhancement (E). Diffusion weighted MR image revealed restricted diffusion suggestive of high cellularity (F). Gradient recovery echo sequence of MR showing absence of blooming (G). Specimen of tumor showing multiple firm, white glistening pieces with punctate brown black pigmentation on cut surface suggestive of melanin (H). |
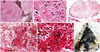 | Fig. 2Histopathology of the tumor. A: Nodules of neuroglial tissue separated by septa with nested pigmented neuroepithelial tubules (H&E, ×40). B: Glial cells and ganglion cells, one binucleate ganglion cell is seen (H&E, ×400). C: Mature hyaline cartilage (H&E, ×400). D: Meningioangiomatosis areas (H&E, ×100). E: Whorling pattern of spindle cells suggestive of meningiomatosis (H&E, ×400). F: Autopsy specimen of brain showing shell of residual tumor anterolaterally. |
References
1. Ajayi O, Palma A, Sadanand V, Deisch J. Pineal anlage tumor: case report and review of literature. JSM Neurosurg Spine. 2014; 2:1035.
2. Fuller GN, Scheithauer BW. The 2007 Revised World Health Organization (WHO) Classification of Tumours of the Central Nervous System: newly codified entities. Brain Pathol. 2007; 17:304–307.

3. Gudinaviciene I, Pranys D, Zheng P, Kros JM. A 10-month-old boy with a large pineal tumor. Brain Pathol. 2005; 15:263–264.

4. Yi JW, Kim HJ, Choi YJ, et al. Successful treatment by chemotherapy of pineal parenchymal tumor with intermediate differentiation: a case report. Cancer Res Treat. 2013; 45:244–249.

5. Schmidbauer M, Budka H, Pilz P. Neuroepithelial and ectomesenchymal differentiation in a primitive pineal tumor ("pineal anlage tumor"). Clin Neuropathol. 1989; 8:7–10.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download