Abstract
Brain metastasis represents one of the most common causes of intracranial tumors in adults, and the incidence of brain metastasis continues to rise due to the increasing survival of cancer patients. Yet, the development of cystic brain metastasis remains a relatively rare occurrence. In this review, we describe the characteristics of cystic brain metastasis and evaluate the combined use of stereotactic aspiration and radiosurgery in treating large cystic brain metastasis. The results of several studies show that stereotactic radiosurgery produces comparable local tumor control and survival rates as other surgery protocols. When the size of the tumor interferes with radiosurgery, stereotactic aspiration of the metastasis should be considered to reduce the target volume as well as decreasing the chance of radiation induced necrosis and providing symptomatic relief from mass effect. The combined use of stereotactic aspiration and radiosurgery has strong implications in improving patient outcomes.
The incidence of brain metastasis in cancer patients is about 20-40%, with about 170,000 new cases every year in the United States [1,2]. Although common, the clinical manifestations have not been well studied. The incidence of brain metastasis is increasing due to the improvement of diagnostic screening tools and treatments, leading to longer life spans of cancer patients [3]. Without intervention, brain metastases have a poor prognosis, with a median survival of only 1 month [4,5,6]. Standard treatments for brain metastasis have not been well established in literature, however some general guidelines are available; surgery, radiosurgery, whole brain radiation therapy (WB-RT) and chemotherapy have proved to be effective.
Cystic brain metastases are relatively rare [7] and the pathogenesis of cyst formation is not clear. According to literature [8] the exudative fluid, often seen as massive edema surrounding the metastatic tumor, tends to collect inside the tumor and expand as a cystic mass depending on its local conditions. Cumings [9] suggested the cyst formation is from the tumor degeneration followed by transudation of fluid from nearby blood vessels. Alternatively, Gardner et al. [10] advocates that the cystic components in brain tumors are merely interstitial fluid without its normal drainage route due to the lack of lymphatics in the surrounding brain.
Large cysts often produce neurologic deficits because of its mass effect. Nevertheless, cystic tumors that cause symptoms must be evaluated and carefully treated. Single, large cystic brain tumors have been traditionally treated through surgery. However, radiosurgery has gained increasing prevalence for the treatment of brain metastasis [11,12,13,14,15,16,17,18] and possesses many advantages over surgical resection. For example, radiosurgery can be used to treat multiple metastases and surgically inaccessible locations such as eloquent or deep areas. In addition, resection is often not a safe choice for patients with severe systemic diseases or advanced age as their physical condition may be poor and unsuitable for general anesthesia [19,20].
However, cystic metastatic tumors themselves are often large and radiosurgery may not be feasible. In this case, the tumors are treated with a decreased radiation dosage to avoid radiation-associated complications [21]. Flickinger [22] reported that tumors with a cystic component greater than 10 mL did not appear to be effectively controlled by radiosurgery alone. Therefore, it is essential to decrease the volume of the cystic components before treating them with radiosurgery. The combination of cyst aspiration and radiosurgery is one possible method [15,23,24,25] that may be more effective and safer than radiosurgery alone.
Large cystic brain metastases share common characteristics with each other. Ebinu et al. [26] analyzed 111 metastatic lesions from 73 patients and reported that lung cancer was the primary cancer in 37 patients (51%) and breast cancer in 7 (10%) patients. Other authors showed similar results with lung cancer reported as the most common origin of brain metastases with breast cancer coming in second [19,27,28]. Cystic change is most common in lung cancer, but also occurs in other metastatic cancers like breast, pancreas, kidney and even melanoma [29]. However, there was a conflicting study that reported breast cancer (50%) as the most common origin and lung cancer (30%) as the second [30]. The distribution of brain metastases varied widely. Some papers did not mention the specific anatomical location of the tumor, but supratentorial lesions are more common than infratentorial lesions [19,27]. Ebinu et al. [26] and Yamanaka et al. [30] showed that the frontal lobe (39%) was the most common site of brain metastases, followed by parietal lobe and cerebellum, but Higuchi et al. [28] reported that the parietal lobe (28%) was the most common site. The mean age for detecting cystic brain metastasis is in the fifties and there is no specific evidence to suggest a preposition for either sex [19,21,27,28,30]. Large cystic brain metastases did not appear to be specific to a particular recursive partitioning analysis (RPA) class but most papers reported class I and II as the most common and class III as the least [RPA index: there are three classes in descending prognostic expectancy from 1 to 3; class 1, for patients with Karnofsky performance status (KPS) 70, <65 years of age, with controlled primary and no evidence of extracranial metastasis; class 3, for patients with KPS <70; class 2 for all others] [19,26,27]. Franzin et al. [19] reported a mean KPS score of 88 (range 70-110) for patients with cystic brain metastases, whereas Yamanaka et al. [30] reported a mean KPS score of 70 (range 50-100). Other authors showed similar results. The volume of cystic metastases, as measured prior to radiosurgery, ranged from 3-100 mL with a mean target volume of 20-25 mL [19,21,27,28].
Most hospitals follow similar protocols for treating cystic metastases. After administering a local anesthetic agent to the head pin sites, a Leksell stereotactic frame is applied using fine adjustments. Magnetic resonance images (MRI) are then obtained to localize the tumors. The path for the aspiration needle is calculated using programs such as the SurgiPlan Electa Instruments system (Elekta, Sweden). The patient is then placed in an appropriate position to deliver the local anesthetic agent and start the stereotactic needle aspiration. The cyst aspiration is done with an aseptic technique. After the drainage, a second set of MRIs is obtained to calculate the reduction in volume. Some hospitals perform the aspiration and the radiosurgery on a same day while other hospitals perform the two procedures within 48 hours of each other. The mean prescription dose to the tumor margin is 17-21 Gy with a range from 12-25 Gy [19,21,26,27,28]. Most hospitals recommend a follow-up MRI every 2 to 3 months post-treatment. Patients are usually discharged on the same day of the procedure or within a few days.
Brain tumor size is an essential factor in determining treatment plans. Studies [4,13,14] show that local tumor control rates of metastases after radiosurgery ranged from 73-94%. The correlation between tumor volumes, prescription dose, and patient survival rates are well known. Schoeggl et al. [31] reported that brain tumors with a maximum diameter less than 17 mm had a better outcome. Sneed et al. [32] noted that a smaller tumor volume was also very closely related to patient survival. Petrovich et al. [33] concluded that tumor volume is an important prognostic factor influencing survival rate by showing that the local tumor control rate one year post-treatment was 90% in tumors <3 mL and 78$ in larger lesions (11 months for tumor volume <1 mL and 6 months for tumor volume >9 mL).
By reducing tumor volume, cystic aspiration aims to accomplish two goals: achieve a better likelihood of tumor control with radiosurgery, and increase the radiosurgery dosage as larger tumor volumes limit the single fraction dose. Franzin et al. [19] reported that all but one patient showed good reduction of the cyst volume after stereotactic drainage. The mean cyst volume was reduced from 21.8 mL to 10.1 mL; the volume reduction was approximately 50.8% (range 5-72%). In his study of 23 patients, 2 (8.7%) presented with tumor progression after radiosurgery, 9 (39.1%) presented with remote progression, and 3 (13%) experienced both. In 5 patients, 7 of the 81 metastases progressed after radiosurgery. Higuchi et al. [28] showed similar results with a mean tumor volume reduction of 20.3 cc to 10.3 cc following aspiration. Good tumor control was obtained in 16 of 21 cases that were evaluated with a median follow-up of 11 months (range 1-27 months). Park et al. [27] showed better results with the mean tumor volume reduced from 23.2 cc (range 7.9-100.9 cc) to 4.3 cc (range 0.2-19.0 cc) with a mean tumor volume reduction of 77.9% (range 31.4-98.3%) (Fig. 1, 2). After treatment, 13 patients (54.2%) showed tumor control, and 11 (45.8%) showed tumor progression with 5 (20.8%) locally and 6 (25.0%) remotely. One patient (4.2%) presented with remote tumor progression and WBRT was required, whereas the other 10 patients (41.7%) needed a second course of radiosurgery. Radiation Therapy Oncology Group 90-05 protocol suggest that tumors with a maximum diameter of 31-40 mm, 21-30 mm and ≤20 mm should receive the maximum tolerated doses of 15, 18, and 24 Gy, respectively [34]. In one study, the volume before drainage was greater than 14 mL (average diameter >30 mm) in 22 (66%) of 33 lesions, which would have given a prescription isodose of <18 Gy. However after drainage, the target volume of 15 out of 22 lesions was <10 mL (average diameter <26 mm) which leads to a prescription isodose >18 Gy [19].
One great advantage of cyst drainage is acute symptomatic relief due to a reduction in mass effect. Liu et al. [21] aspirated cystic metastatic tumor from 43 of 77 patients (excluding tumors that were glial, vestibular schwannomas, etc.) and found that 68 patients (88.3%) showed immediate improvement of symptoms following drainage. In the study, 62 patients had a follow-up for at least 6 months and 5 patients showed disappearance of the metastatic cystic tumor. Another study [27] which treated patients with radiosurgery after stereotactic cyst drainage, found that 19 out of 24 patients (79.2%) showed improvement in symptoms related to their brain metastasis. Furthermore, Niranjan et al. [24] reported on the importance of tumor location for symptomatic relief. In his study, 11 of 13 patients (77%) with cystic tumors in deep locations (brain stem, hypothalamus, and thalamus) noted symptomatic improvement. Of the patients with cysts in a lobar location, 17 of 25 (68%) experienced improvement following cyst aspiration. Thus, these studies support the increase in feasibility and efficacy of Gamma Knife Surgery in improving patient outcomes.
Franzin et al. [19] reported a local tumor control rate of 91.3% and all cystic tumors except for 2 were controlled at the end of follow-up (mean follow-up of 11.3 months, with a median of 9 months). The median time of tumor progression was 10 months. In one study [28], 16 of the 21 patients (76%) had tumors that were controlled well at the end of follow-up or at the time of the patient's death from non-neurological causes (median following up 11 months). During follow-up, 19 patients died, 1 patient remained alive, and 5 patients were lost to follow-up. Of the 19 patients who died, only 3 patients displayed significant neurological progression. Gerosa et al. [14] showed 93% local tumor control in 804 patients with brain metastases, 15 of which were cystic lesions. Another study [27] revealed that only 1 patient (4.2%) died from the progression of brain metastasis while 3 patients (12.5%) died from unrelated illnesses and 6 patients (25.0%) died from primary cancer progression (mean follow-up 13.1 months). Median progression-free survival after treatment was 14.1 months. Other authors reported a median survival of 7.5 months in 8 patients [25] and 26.6 months in 15 patients [14] with a 1-year actuarial survival rate of 9%. Reaccumulation of cyst contents was observed after the aspiration. In a study by Park et al. [27], 12 lesions (48.0%) were treated with additional Ommaya reservoir insertion after stereotactic drainage. Higuchi et al. [28] had reaccumulation of cyst contents in 2 patients (9%) who required Ommaya reservoir placement as well. Intracystic hemorrhage associated with Ommaya reservoir placement was seen in four tumors (16%) in two patients [30]. These tumors did not shrink, but no neurological deterioration was observed.
Severe acute complications related to the aspiration or radiosurgery are not frequently observed. However, possible complications include neurosurgical deficits, hemorrhage, seizures, and infections [35]. The mortality rate in several large series has been less than 1% and complication rates vary from 0% to 7% [35,36]. Approximately one-third of patients experienced mild transitory symptoms, including headache, nausea, and dizziness following radiosurgery [37]. More severe complications such as facial palsy, trigeminal neuropathy, and visual symptoms occur 6 to 9 months after the procedure [38]. The incidence of radiation necrosis was 6.6% in one study and no events of radiation necrosis were found in lesions with volumes less than 15 mL. This suggests that reducing treatment volume to less than 15 mL will reduce complications due to radiation included necrosis [19]. However, if the tumor volume remains large even after drainage, fractionated radiosurgery can be used to mitigate the risk of necrosis by reducing the volume of normal brain receiving a single, high dose fraction [39]. There is also the theoretical risk for tumor cell seeding along the aspiration needle tract, but the incidence of such events are poorly described in literature. To date, these rare complications have only occurred after stereotactic biopsy of pineoblastoma, craniopharyngioma, anaplastic astrocytoma, and certain intracranial metastasis [40,41,42,43].
There are many studies that investigated the prognostic factors for patient survival and tumor control after radiosurgery. In one study [19], the extension of the extracranial illness (p=0.001), male sex (p=0.02) and different tumor types (p=0.006) were statistically significant prognostic factors. A statistically significant correlation between tumor volume and the prescription radiation dose and final tumor control rate was not found [19]. Tendulkar et al. [44] concluded lung cancer (p=0.02) as the subtype identified as a positive predictor and prior WBRT (p=0.03) as a less favorable response to radiosurgery. The most important prognostic value was the RPA classification (p<0.001); the survival rate of patients included in RPA class I (median 25 months) was higher than that in RPA class II (median 8 months). The importance of RPA classification has been confirmed in other studies as well. Lutterbach et al. [45] showed median survival rates of 13.4, 9.3, and 1.5 months in RPA classes I, II, and III, respectively; Sneed et al. [46] found a median survival rate of 14 months for patients in RPA class I and 8.2 months for patients in RPA class II. Another study [27] also reported an overall median survival after radiosurgery of 17.8 months (range 1-39 months); 17.8 months for patients in RPA class I, 10.9 months for patients in RPA class II, and 6.1 months for patients in RPA class III. Therefore, RPA classifications can be used as a valid tool for predicting prognosis in patients with brain metastases. Patients in RPA class III are typically not healthy enough to permit general anesthesia for surgery, but the great advantage of radiosurgery is that this modality can be performed using only local anesthesia. This reinforces the efficacy of radiosurgery after stereotactic cyst aspiration for inaccessible cystic lesions on a wide range of patient types [27].
The aim of this review is to evaluate and assess the use of combining stereotactic drainage in cystic metastases tumors in improving the efficacy and feasibility of radiosurgery. The results of the cited studies show great potential in the non-surgical management of cystic metastases. There have been no direct, randomized clinical comparisons between radiosurgery and other surgical-radiation protocols, but it has been shown that patients with single lesions can achieve similar or better results with radiosurgery when compared to those who were treated with other protocols [22,47,48,49,50]. Stereotactic radiosurgery is a non-invasive and effective treatment tool when measured in terms of local tumor control and patient survival rates. Surgery and radiosurgery should not be considered as separate treatment techniques, but rather complementary. When radiosurgery is not efficient due to the size of the tumor, the aspiration of cystic metastases can be considered an option. Aspiration allows the radiation dose to be reduced to the target volume, thereby decreasing the chance of radiation induced necrosis or other complications. Therefore, it is possible to deliver a higher prescription dose to the tumor while also promoting acute symptoms relief from the decreased mass effect. The available literature shows that the combined usage of stereotactic aspiration and radiosurgery has the potential ability to improve patient outcomes.
Figures and Tables
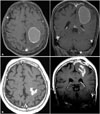 | Fig. 1Axial and coronal contrast-enhanced T1-weighted magnetic resonance images of the brain of a 66-year-old woman with a large cystic brain metastasis, with hemorrhage, that developed from non-small lung cancer. A: Before aspiration the initial cyst volume was 25.5 cc. B: After cyst aspiration, cyst volume was 5.5 cc. The prescription dose was 20 Gy and MR shows 6 years after postoperatively. |
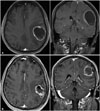 | Fig. 2Axial and coronal contrast-enhanced T1-weighted magnetic resonance images of the brain of a 73-year-old woman with a large cystic brain metastasis, that developed from breast cancer. A: Before aspiration the cyst volume was 43.2 cc. B: After cyst aspiration, cyst volume was 18.9 cc. The prescription dose was 18 Gy and MR shows about 10 postoperative months. |
Acknowledgments
Isaac Yang (senior author) was partially supported by a Visionary Fund Grant, an Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research UCLA Scholars in Translational Medicine Program Award, the Jason Dessel Memorial Seed Grant, the UCLA Honberger Endowment Brain Tumor Research Seed Grant, and the STOP CANCER Research Career Development Award.
References
2. Patchell RA, Regine WF. The rationale for adjuvant whole brain radiation therapy with radiosurgery in the treatment of single brain metastases. Technol Cancer Res Treat. 2003; 2:111–115.

3. Wen PY, Loeffler LS. Management of brain metastases. Oncology. 1999; 13:941–954.
4. Hazuka MB, Burleson WD, Stroud DN, Leonard CE, Lillehei KO, Kinzie JJ. Multiple brain metastases are associated with poor survival in patients treated with surgery and radiotherapy. J Clin Oncol. 1993; 11:369–373.

5. Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999; 43:795–803.

6. Markesbery WR, Brooks WH, Gupta GD, Young AB. Treatment for patients with cerebral metastases. Arch Neurol. 1978; 35:754–756.

7. Andrews RJ, Gluck DS, Konchingeri RH. Surgical resection of brain metastases from lung cancer. Acta Neurochir (Wien). 1996; 138:382–389.

8. Hossmann KA. Development and resolution of ischemic brain swelling. Wien, New York: Springer-Verlag;1976. p. 249–258.
10. Gardner WJ, Collis JS Jr, Lewis LA. Cystic brain tumors and the blood-brain barrier. Comparison of protein fractions in cyst fluids and sera. Arch Neurol. 1963; 8:291–298.
11. Alexander E 3rd, Moriarty TM, Davis RB, et al. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst. 1995; 87:34–40.

12. Boyd TS, Mehta MP. Stereotactic radiosurgery for brain metastases. Oncology (Williston Park). 1999; 13:1397–1409.
13. Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994; 28:797–802.

14. Gerosa M, Nicolato A, Foroni R, et al. Gamma knife radiosurgery for brain metastases: a primary therapeutic option. J Neurosurg. 2002; 97:5 Suppl. 515–524.

15. Loeffler JS, Barker FG, Chapman PH. Role of radiosurgery in the management of central nervous system metastases. Cancer Chemother Pharmacol. 1999; 43:S11–S14.

16. Mehta M, Noyes W, Craig B, et al. A cost-effectiveness and cost-utility analysis of radiosurgery vs. resection for single-brain metastases. Int J Radiat Oncol Biol Phys. 1997; 39:445–454.

17. Nakagawa K, Tago M, Terahara A, et al. A single institutional outcome analysis of Gamma Knife radiosurgery for single or multiple brain metastases. Clin Neurol Neurosurg. 2000; 102:227–232.

18. O'Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O'Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003; 55:1169–1176.
19. Franzin A, Vimercati A, Picozzi P, et al. Stereotactic drainage and Gamma Knife radiosurgery of cystic brain metastasis. J Neurosurg. 2008; 109:259–267.

20. Reda WA, Hay AA, Ganz JC. A planned combined stereotactic approach for cystic intracranial tumors. Report of two cases. J Neurosurg. 2002; 97:5 Suppl. 610–612.

21. Liu X, Yu Q, Zhang Z, et al. Same-day stereotactic aspiration and Gamma Knife surgery for cystic intracranial tumors. J Neurosurg. 2012; 117:Suppl. 45–48.

22. Flickinger JC. Radiotherapy and radiosurgical management of brain metastases. Curr Oncol Rep. 2001; 3:484–489.

23. Kim MS, Lee SI, Sim SH. Brain tumors with cysts treated with Gamma Knife radiosurgery: is microsurgery indicated. Stereotact Funct Neurosurg. 1999; 72:Suppl 1. 38–44.

24. Niranjan A, Witham T, Kondziolka D, Lunsford LD. The role of stereotactic cyst aspiration for glial and metastatic brain tumors. Can J Neurol Sci. 2000; 27:229–235.

25. Uchino M, Nagao T, Seiki Y, Shibata I, Terao H, Kaneko I. [Radiosurgery for cystic metastatic brain tumor]. No Shinkei Geka. 2000; 28:417–421.
26. Ebinu JO, Lwu S, Monsalves E, et al. Gamma knife radiosurgery for the treatment of cystic cerebral metastases. Int J Radiat Oncol Biol Phys. 2013; 85:667–671.

27. Park WH, Jang IS, Kim CJ, Kwon do H. Gamma knife radiosurgery after stereotactic aspiration for large cystic brain metastases. J Korean Neurosurg Soc. 2009; 46:360–364.

28. Higuchi F, Kawamoto S, Abe Y, Kim P, Ueki K. Effectiveness of a 1-day aspiration plus Gamma Knife surgery procedure for metastatic brain tumor with a cystic component. J Neurosurg. 2012; 117:17–22.

29. Willis RA. Secondary tumors. In : Minckler J, editor. Pathology of the Nervous System. New York: McGraw-Hill;1971. p. 2178–2196.
30. Yamanaka Y, Shuto T, Kato Y, et al. Ommaya reservoir placement followed by Gamma Knife surgery for large cystic metastatic brain tumors. J Neurosurg. 2006; 105:79–81.

31. Schoeggl A, Kitz K, Ertl A, Reddy M, Bavinzski G, Schneider B. Prognostic factor analysis for multiple brain metastases after gamma knife radiosurgery: results in 97 patients. J Neurooncol. 1999; 42:169–175.
32. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary. Int J Radiat Oncol Biol Phys. 1999; 43:549–558.

33. Petrovich Z, Yu C, Giannotta SL, O'Day S, Apuzzo ML. Survival and pattern of failure in brain metastasis treated with stereotactic gamma knife radiosurgery. J Neurosurg. 2002; 97:5 Suppl. 499–506.

34. Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000; 47:291–298.

35. Lunsford LD, Martinez AJ. Stereotactic exploration of the brain in the era of computed tomography. Surg Neurol. 1984; 22:222–230.

36. Bernstein M, Parrent AG. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg. 1994; 81:165–168.

37. Werner-Wasik M, Rudoler S, Preston PE, et al. Immediate side effects of stereotactic radiotherapy and radiosurgery. Int J Radiat Oncol Biol Phys. 1999; 43:299–304.

38. Kondziolka D, Firlik AD, Lunsford LD. Complications of stereotactic brain surgery. Neurol Clin. 1998; 16:35–54.

39. Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011; 6:48.

40. Barloon TJ, Yuh WT, Sato Y, Sickels WJ. Frontal lobe implantation of craniopharyngioma by repeated needle aspirations. AJNR Am J Neuroradiol. 1988; 9:406–407.
41. Karlsson B, Ericson K, Kihlström L, Grane P. Tumor seeding following stereotactic biopsy of brain metastases. Report of two cases. J Neurosurg. 1997; 87:327–330.
42. Perrin RG, Bernstein M. Iatrogenic seeding of anaplastic astrocytoma following stereotactic biopsy. J Neurooncol. 1998; 36:243–246.
43. Rosenfeld JV, Murphy MA, Chow CW. Implantation metastasis of pineoblastoma after stereotactic biopsy. Case report. J Neurosurg. 1990; 73:287–290.
44. Tendulkar RD, Liu SW, Barnett GH, et al. RPA classification has prognostic significance for surgically resected single brain metastasis. Int J Radiat Oncol Biol Phys. 2006; 66:810–817.

45. Lutterbach J, Cyron D, Henne K, Ostertag CB. Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery. 2003; 52:1066–1073.

46. Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002; 53:519–526.

47. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322:494–500.

48. Mandell L, Hilaris B, Sullivan M, et al. The treatment of single brain metastasis from non-oat cell lung carcinoma. Surgery and radiation versus radiation therapy alone. Cancer. 1986; 58:641–649.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download