Abstract
Intracranial chondroma is a rare benign tumor. Here, we present the case of a 29-year-old female who was afflicted with left eye blindness and ptosis. Brain computerized tomography and magnetic resonance imaging revealed the presence of a giant calcified mass accompanied by a solid mass in the middle and posterior fossa. A differential diagnosis regarding chordoma, chondrosarcoma, and other chondroid tumors based on radiologic information was inconclusive. The lesion was resected completely under a microscope using a combined pterional and subtemporal approach. The pathologic report confirmed the diagnosis of chondroma. No evidence of neurological worsening was observed. The tumor had a calcified mass with mature hyaline cartilage surrounded by a thick fibrous capsule. We dissected the periphery of the tumor mass and removed it via aspiration. It was readily distinguished from normal brain parenchymal tissue. The large calcified mass at the center of the tumor had relatively high vascularity, and a high-speed drill and various rongeurs were used to remove the tumor.
Chondromas are benign tumors arising from the long bones, pelvis, or scapulae. Intracranial chondromas, however, are extremely rare [1,2]. An intracranial chondroma was first reported by Hirschfield in 1851. These lesions usually arise at the base of the skull from embryonic chondrocytic cell remnants [2], but may also originate in the falx, and convexity dura [1,3,4,5,6]. In our case, a giant chondroma with massive calcification was located in the midline parasellar area and extended to the posterior fossa, compresseing the brain stem. Here, we report on the complete surgical resection of a giant skull base chondroma and present a review of the literature [7].
A 29-year-old female presented with six years' of left-side ptosis and blindness. She had been diagnosed with a brain tumor at age 23 and surgery was recommended at that time, but the patient was concerned about postoperative complications and refused the procedure. The patient's visual acuity in her left eye worsened and she subsequently lost her vision in that eye and could not open it. A neurological examination revealed the patient had right hemiparesis (grade 4) and that the left pupil was fully dilated with no reflex response to light and the eyeball was fixed (Fig. 1). A general physical examination revealed left-side ptosis. The results of laboratory testing, including a pituitary function test, were unremarkable. A computed tomography (CT) scan revealed a dense calcification in the central portion of the mass (Fig. 2A, B). Brain magnetic resonance imaging (MRI) showed a lobular non-enhancing mass of 7.0×4.9×5.3 cm in the center of the base of the skull which extended to the prepontine cistern and suprasellar cistern (Fig. 2C-E). The cerebral angiogram showed no vascular abnormalities. The mass was initially diagnosed as a chordoma or chondroid tumor. Based on these results, surgical exploration and excision of the mass was proposed.
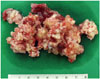
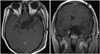
The patient underwent extradural clinoidectomy and tumor mass removal via a combined left pterional and subtemporal approach using image-guided navigation. After the sylvian dissection, a dark-gray color mass was encountered. The large calcified mass at the center of the tumor had relatively high vascularity, so a drill and various rongeurs were used to remove the tumor (Fig. 3). Bone invasion was minmal and limited to sellar floor. After total removal of tumor, a small dural defect occurred in the sellar region; we closed it with tachocomb and gelfoam (Fig. 4). However, after 2 weeks, cerebrospinal fluid (CSF) rhinorrhea occurred. It did not respond to lumbar drainage, so we used the transsphenoidal approach and applied a fascia lata graft to the dura defect, which stopped the CSF rhinorrhea.
Postoperatively, the patient's right hemiparesis improved to normal and while her other neurological status did not change. Postoperative pituitary function was normal. After 2 years, an MRI showed no evidence of reccurence, and the patient had no other clinical symptoms.
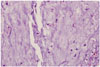
Microscopic findings revealed the proliferation of polygonal to stellate cells in lobules on a myxochondroid background (Fig. 5). There was no nuclear atypism or pleomrophism, and no evidence of host bone entrapment and open chromatin. Differential diagnosis included chondroma, chondrosarcoma, and chordoma. Immunohistochemistry disclosed a positive finding for S-100 protein and negative for cytokeratin and epithelial membrane antigen with very low Ki-67 proliferation index of less than 1%. According to these results, the lesion was finally diagnosed as chondroma.
Russell and Rubinstein [8] reported that the aberrant growth of cartilage rests could cause chondroma in parts of the intracranial cavity other than the base of the skull. Intracranial chondroma can arise from the dura mater, brain parenchyma, ventricle, or skull base. About 70% of reported cases originate from the dura mater, while the remaining cases are located inside the brain parenchyma or the choroid plexus [9]. Clinical features of intracranial chondromas are their slowgrowth and large size at presentation. Accordingly, patients present with a long-standing history of signs and symptoms [10].
The radiologic differential diagnosis of meningioma, low-grade chondrosarcoma, and chordoma is essential. Angiography may be the best diagnostic method for differentiating between chondroma and meningioma. Chondromas are avascular, whereas meningiomas typically exhibit a late-capillary and early-venous tumor blush from the meningeal arterial supply. CT scans reveal a well-circumscribed mass with lesional calcification, accompanied by erosion and destruction of the surrounding bone [2]. MRIs of intracranial chondromas show peripheral hypointensity relative to brain parenchyma on T1- and T2-weighted images that represent mature hyaline cartilage. The lack of peritumoral edema indicates slow growth over a period of many years.
Another differential diagnosis is low-grade chondrosarcoma, one of the most difficult subjects of surgical pathology. Shariat Torbaghan et al. [11] found that lobulation patterns and fibrous tissue formation around the tumor can be effective indicators for differentiation. In our case, there was no regular cartilage lobule with mature connective tissue. There was no histological evidence that the tumor margin was surrounded by mature lamellar bone or normal marrow cells. Additionally, binuclear chondrocytes were abundant. These factors suggest that the tumor in this case was a low-grade chondrosarcoma. However, another study applied different criteria. Eefting et al. [12] showed that a combination of five parameters (high cellularity, presence of host bone entrapment, open chromatin, mucoid matrix quality, and age above 45 years) allowed optimal differentiation between enchondromas and central grade 1 chondrosarcomas. In our case, four parameters suggest benign chondroma. Our patient's tumor did not have a mucoid matrix quality. Skull base chondroma is very rare, so its histopathologic features could be different from those of long bone chondrogenic tumors. Despite the histopathologic controversy between chondroma and low grade chondrosarcoma, we concluded that this tumor was chondroma, because there was no evidence of recurrence after 2 years, which weighs in favor of the benign tumor.
This case had a calcified mass with mature hyaline cartilage surrounded by a thick fibrous capsule. The author was able to perform intracapsular debulking using various rongeurs and a high-speed drill followed by removal of the tumor capsule from the brain parenchyma in a relatively safe manner, considering the size of the tumor. However, no boundary existed among the central calcified mass, dorsum sellae, and clivus. Thus, careful handling was necessary while removing the calcified mass around the cavernous sinus, dorsum sellae, and clival areas.
Complete resection is the treatment of choice when the lesion is amenable to total removal. Radiation therapy is not effective for chondroma and a malignant transformation to chondrosarcoma has been reported after partial resection [13]. Local invasion or recurrence may identify an instance of malignant degeneration into chondrosarcoma. No recurrence is expected following complete resection of the tumor, and the long-term prognosis is good [14,15,16].
In conclusions, intracranial chondroma is a rare benign neoplasm. Surgical excision is the treatment of choice. In our case, there was a calcified cartilaginous mass with a thick capsule. We found that intracapsular debulking using various rongeurs and a high-speed drill followed by the complete removal of the tumor capsule from the brain parenchyma to be relatively safe for a large tumor. However, no boundary existed among the central calcified mass, dorsum sellae, and clivus. Thus, careful handling was necessary while removing the calcified mass around the cavernous sinus, dorsum sellae, and clival areas.
Figures and Tables
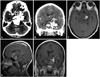 | Fig. 2Computed tomography (CT) revealed a giant high density lesion in the central protion of the mass indicating dense calcification (A and B). Gadolinium-enhanced axial magnetic resonance imaging (MRI) revealed a solitary mass in the prepontine cistern extending to the suprasellar cistern. The lesion showed heterogeneous nonenhancement following the intravenous administration of a gadolinium-based contrast agent. No evidence of peritumoral edema was observed (C, D, and E). |
References
1. Kurt E, Beute GN, Sluzewski M, van Rooij WJ, Teepen JL. Giant chondroma of the falx. Case report and review of the literature. J Neurosurg. 1996; 85:1161–1164.
2. Patel A, Munthali L, Bodi I. Giant cystic intracranial chondroma of the falx with review of literature. Neuropathology. 2009; 29:315–317.

3. Pallini R, Lauretti L, Fernandez E, Colosimo C. Giant chondroma of the falx. J Neurosurg. 1997; 87:333–334.
4. Mobbs RJ, Narula S, Berger M, Kwok BC. Intracranial chondroma of the occipital lobe. Australas Radiol. 1998; 42:74–76.

5. Nakazawa T, Inoue T, Suzuki F, Nakasu S, Handa J. Solitary intracranial chondroma of the convexity dura: case report. Surg Neurol. 1993; 40:495–498.

6. De Coene B, Gilliard C, Grandin C, Nisolle JF, Trigaux JP, Lahdou JB. Unusual location of an intracranial chondroma. AJNR Am J Neuroradiol. 1997; 18:573–575.
7. Ramamurthi B, Iyer CG, Vedachalam SP. Intracranial meningeal chondroma. J Neurosurg. 1961; 18:826–828.

8. Russell DS, Rubinstein LS. Pathology of Tumours of the Nervous System. 2nd ed. London: Edward Arnoid Ltd.;1963.
9. Lacerte D, Gagné F, Copty M. Intracranial chondroma. Report of two cases and review of the literature. Can J Neurol Sci. 1996; 23:132–137.

10. Nakayama M, Nagayama T, Hirano H, Oyoshi T, Kuratsu J. Giant chondroma arising from the dura mater of the convexity. Case report and review of the literature. J Neurosurg. 2001; 94:331–334.
11. Shariat Torbaghan S, Ashouri M, Jalayer Naderi N, Baherini N. Histopathologic Differentiation between Enchondroma and Well-differentiated Chondrosarcoma: Evaluating the Efficacy of Diagnostic Histologic Structures. J Dent Res Dent Clin Dent Prospects. 2011; 5:98–101.
12. Eefting D, Schrage YM, Geirnaerdt MJ, et al. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol. 2009; 33:50–57.

13. Grossman RI, Davis KR. Cranial computed tomographic appearance of chondrosarcoma of the base of the skull. Radiology. 1981; 141:403–408.

14. Acampora S, Troisi F, Fusco G, Del Gaizo S. Voluminous intracranial chondroma. Surg Neurol. 1982; 18:254–257.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download