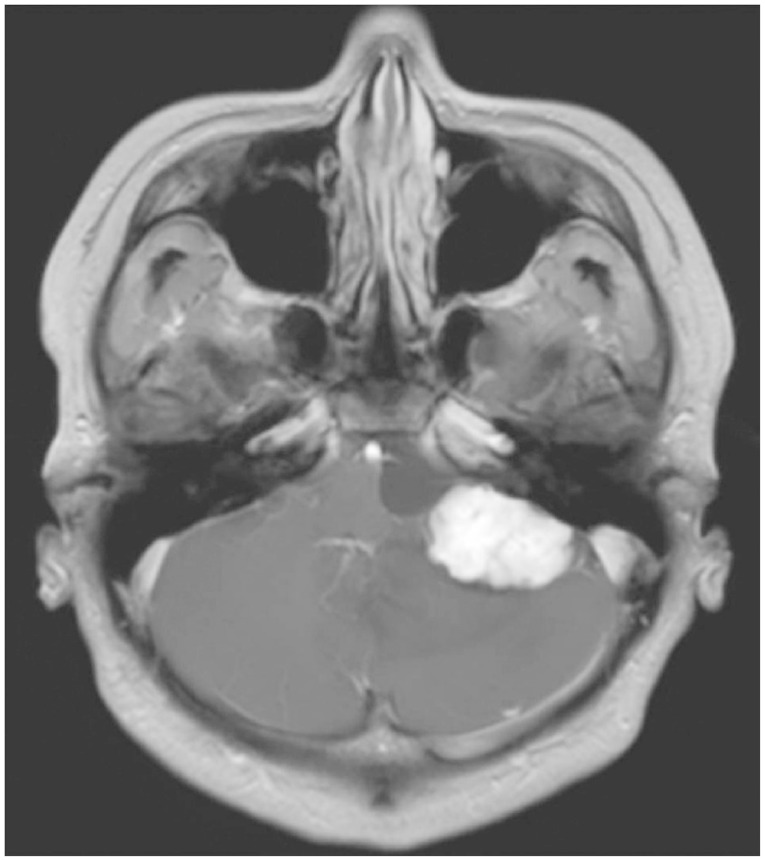
Hemangioblastomas of the posterior fossa typically arise within the substance of the cerebellum and the brainstem [
2]. However, unusual HBMs involving the cranial nerve and the CPA have rarely been reported [
2,
3,
4,
5,
7,
8,
9,
10]. HBMs are generally intra-axial in origin, so the CPA is very rarely involved [
2,
3,
4,
5,
7,
8,
9]. In English literature, only 10 cases of CPA HBM have been reported [
2,
7]. In this case, the tumor was placed in the CPA. However, we identified the tumor attached to the surface of the cerebellum around the foramen of Luschka. Therefore, that areas was seemingly the site of origin. In our opinion, the tumor derived from the cerebellar pial vessels was intra-axial in origin. However, the tumor growth was directed into the CPA, an extra-axial location, and not into the cerebellar parenchyme. Although 6-10% of all intracranial tumors were found in the CPA, most of them are VSs and meningiomas [
2,
3,
4]. Because of the differences in the surgical strategies used for these tumors, differential diagnosis is crucial to the safe management of HBMs. Classically, about two-thirds of HBMs appear as well-circumscribed cystic masses with hypervascular mural nodules [
2]. Radiological findings of HBMs show multiple signal voids in the lesion, a peritumoral cyst, and peritumoral edema. Although there are no histological differences between the cystic and solid tumor subtypes [
9], solid subtypes have been regarded as difficult to treat surgically because of their AVM-like characteristics [
4,
7,
9]. Usually, after aspiration of the cystic fluid, sufficient exposure for surgery can be achieved in cystic HBMs. However, solid subtypes may require a more extended approach to achieve an adequate work space, not only due to their solid nature but also due to their AVM-like character. Therefore, we used modified TCFA. Various approaches that included the retrosigmoid [
5,
7,
9], translabyrinthine [
2], and transcochlear approaches [
4] have been used with or without pre-operative embolization. Dow et al. [
4] used the trans-cochlear far lateral approach in patients with large (>3 cm) solid HBMs in the CPA. This approach provided a wide enough exposure to achieve early control of proximal feeding vessels and to dissect the lesion circumferentially [
4]. For cystic HBMs in the CPA, Bush et al. [
2] removed the tumor via the translabyrinthine approach because they first thought the lesion was an atypical cystic VS. They suggested that had an HBM been considered, the suboccipital approach might have provided adequate exposure and potentially preserved hearing [
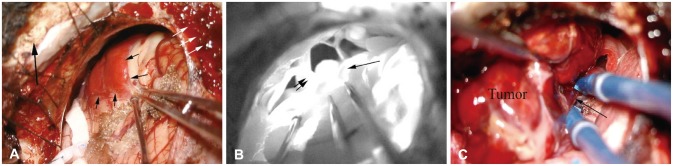
2]. To achieve adequate exposure, we used modified TCFA. Compared to retrosigmoid craniotomy, TCFA extended the bony opening to approach the midline lower clival lesion by drilling the bone over the condylar fossa and the jugular tubercle [
6]. For midline access in the CPA, the jugular tubercle should be partially resected in the conventional TCFA [
6]. However, in our case, the tumor already made the space. Therefore, careful approach of the proximal feeding vessels such as AICA and their early control were possible without resection of the jugular tubercle. The more significantly extended bony removal provided a wider space for the circumferential dissection. To reduce the vascularity of the tumor, pre-operative embolization [
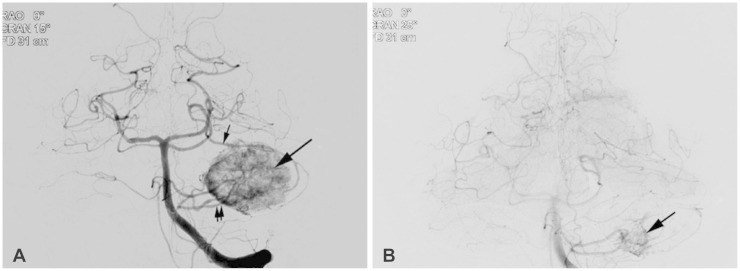
11] was carried out in our case. To reduce the vascularity, Kamitani et al. [
5] used pre-operative radiosurgery in a 3.5 cm-diameter solid hypervascular HBM. They reported that radiosurgery nine months before craniotomy significantly reduced the vascularity and subsequently enabled complete and safe tumor removal [
5]. Solid HBMs are minor subtypes that mimic AVMs, and for which a careful strategy that includes pre-operative embolization and/or radiosurgery should be considered, besides a tailored approach, as with surgery on AVMs.
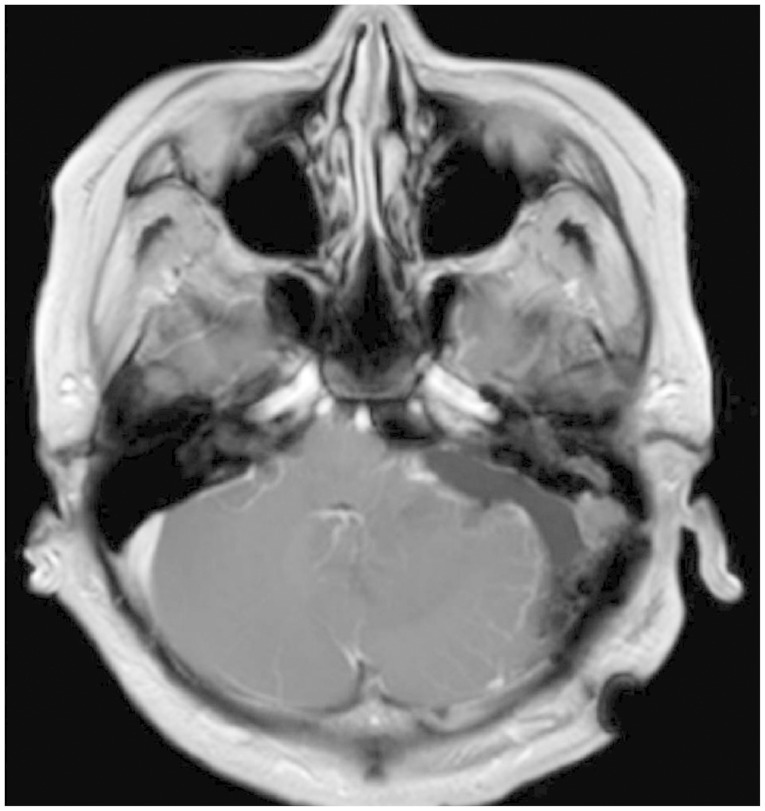
In conclusion, HBMs can present within the CPA, mimicking other pathologies such as VS and meningioma. Although unusual, HBMs should be considered in differential diagnosis of CPA tumors. Large solid HBM subtypes are similar in clinical character to intracranial AVMs. Therefore, for the safe resection of the lesion, a specific strategy for reducing the vascularity of the tumor and the tailored skull base approach should be considered.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download