Abstract
Glioblastoma multiforme (GBM) is well known as the most common malignant primary brain tumor. It could easily spread into the adjacent or distant brain tissue by infiltration, direct extension and cerebro-spinal fluid dissemination. The extranueural metastatic spread of GBM is relatively rare but it could have more progressive disease course. We report a 39-year-old man who had multiple bone metastases and malignant pleural effusion of the GBM without primary site recurrence.
Distant metastasis of glioblastoma multiforme (GBM) is well known; most commonly through cerebro-spinal fluid flow [1,2]. But metastasis of GBM to the outside of the central nervous system (CNS) is rare. The incidence of extraneural metastasis of GBM was reported as 0.2% by Hsu et al. [3], but is increasing. The most common site of metastasis to the outside of the CNS was known as pleura/lung, lymph nodes and bones [4,5]. Most of these cases had multiple metastases with primary site progression. We report a case of multiple bone metastases and malignant pleural effusion of GBM without primary site recurrence.
A 39-year-old man presented with 2 weeks history of headache and visual disturbance. Magnetic resonance studies showed a huge multi-lobulated contoured necrotic enhancing mass in right parieto-occipital area (Fig. 1A). Under the presumptive diagnosis of high grade glioma, craniotomy and tumor removal was performed, and residual tumor was not observed on initial postoperative magnetic resonance images (Fig. 1B). The pathological diagnosis was GBM (Fig. 2A). The patient was treated using three-dimensional conformal radiotherapy (6,000 cGy, 240 cGy, 25 fraction) combined with temozolomide (TMZ, 75 mg/m2/day), and then 4 cycles of standard adjuvant TMZ (150 mg/m2/day during five days).
Sudden severe back pain and lower extremity paraplegia occurred seven months after initial diagnosis of GBM. Spine magnetic resonance imaging (MRI) revealed compression fracture of sixth thoracic vertebra and epidural mass compressing spinal cord (Fig. 3), but there was no intracranial recurrence in brain MRI (Fig. 1C). Emergency decompressive laminectomy and epidural mass excision were performed. The pathologic diagnosis was suggestive of metastatic lesion of GBM (Fig. 2B). Additional multiple bone metastases in T-L spine, both ribs, both pelvic bones and right femur shaft were founded through whole body positron emission tomography and bone scan (Fig. 4). Also suspicious metastatic tumor cells were detected from pleural fluid. Excisional biopsy of the osteomyelitis-like lesion at head of left fourth rib proved it another metastatic GBM (Fig. 2C). The patient had uncontrolled malignant pleural effusion and rapid systemic aggravation. In spite of active mechanical ventilation and best supportive care in intensive care unit, he expired three months later.
Glioblastoma multiforme is one of the most common malignant neoplasms which commonly spreads directly to adjacent brain tissue along the white matter tract. In spite of remarkable local invasiveness, the extraneural metastasis of the GBM is extremely rare. Piccirilli et al. [4] reported that most common metastatic site was lung (n=44, 34.4%), and then bone (n=29, 22.9%) at which affected vertebrae most frequently, among 128 patients with extra CNS metastasis in the English literature.
The scarcity of metastases from GBM could not have been explained clearly, but some reasons have been proposed. First reason is that the short life span of patient with GBM eliminates the chance of detectable metastases of GBM [6]. The second reason is blood-brain barrier (BBB) plays an important role physically against migration of the brain tumor cells into the blood stream [7]. The other reasons are that the deficiency of extracellular matrix component and lymphatic system in brain makes tumor cell difficult to metastasize to extraneural spaces.
The risk factors of extraneural metastasis of GBM were not clear. But many of neuro-oncologist has agreed that young age, prolonged survival time, repeated recurrence, high grade tumor histology and sarcomatous component are related to extraneural metastases [7,8]. It is widely accepted that neurosurgical operation associated with the opening of the brain vessels could damage BBB, which make haematogenous extraneural spreading of brain tumor. Huang et al. [8] reported that nearly all (96%) of patients with extraneural metastases of GBM underwent neurosurgical procedures beforehand.
Most of reported cases of metastatic GBM have primary brain tumor recurrence, only a few cases have been reported where extracranial metastases from GBM occurred without any relapse in the brain. In present case, although the primary brain GBM was well controlled, rapid progression of metastases of GBM make him die. Considering short period of extraneual metastases of the patient after single neurosurgical operation, initially concurrent systemic metastases of GBM can not be excluded.
In conclusion, extraneural metastasis of GBM is very rare, but the prognosis of metastatic GBM could be very poor even though primary brain tumor is well controlled. So, appropriate systemic evaluation of patient of malignant brain tumor has to be considered. And more studies about the risk evaluations for extraneural metastasis are needed.
Figures and Tables
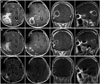 | Fig. 1A: Preoperative magnetic resonance imaging (MRI) reveals huge multi-lobulated contoured necrotic enhancing mass in right occipital lobe with extensive peritumoral edema. B: Postoperative MRI after one day-most of the enhancing mass are removed. C: Follow-up MRI at 10 months after surgery shows no evidence of tumor recurrence. |
 | Fig. 2Histologic findings of tumor mass from brain (A), spine (B), and rib (C) show pleomorphic astrocytic tumor cells with mitosis and nuclear atypia compatible to glioblastoma (hematoxylin-eosin, original magnification, ×400). |
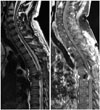 | Fig. 3Spine magnetic resonance imaging at 7 months after initial surgery reveals compressive pathologic fracture at the 6th thoracic vertebral body. |
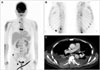 | Fig. 4A: Whole body fluorodeoxyglucose positron emission tomography scan imaging reveals multiple bone metastases to spine, ribs, pelvic, and scrum. B: Bone scan imaging shows multiple bone metastases in T-L spine and both ribs. C: Chest CT image reveals both malignant pleural effusion and both plural nodularity with focal chest wall invasion. |
References
1. Shah A, Redhu R, Nadkarni T, Goel A. Supratentorial glioblastoma multiforme with spinal metastases. J Craniovertebr Junction Spine. 2010; 1:126–129.

2. Vertosick FT Jr, Selker RG. Brain stem and spinal metastases of supratentorial glioblastoma multiforme: a clinical series. Neurosurgery. 1990; 27:516–521. discussion 521-2.

3. Hsu E, Keene D, Ventureyra E, et al. Bone marrow metastasis in astrocytic gliomata. J Neurooncol. 1998; 37:285–293.
4. Piccirilli M, Brunetto GM, Rocchi G, Giangaspero F, Salvati M. Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients. Clinico-pathological remarks on our series of seven cases and critical review of the literature. Tumori. 2008; 94:40–51.

5. Rajagopalan V, El Kamar FG, Thayaparan R, Grossbard ML. Bone marrow metastases from glioblastoma multiforme--a case report and review of the literature. J Neurooncol. 2005; 72:157–161.

6. Mentrikoski M, Johnson MD, Korones DN, Scott GA. Glioblastoma multiforme in skin: a report of 2 cases and review of the literature. Am J Dermatopathol. 2008; 30:381–384.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download