Abstract
Although uncommon, hemorrhage can be a complication of low grade glioma with an unfavorable prognosis such as transformation to higher grade glioma. To our knowledge, hemorrhagic recurrence of World Health Organization Grade II, diffuse astrocytoma without malignant transformation has not been reported. Thus, we report a case of diffuse astrocytoma with hemorrhagic recurrence without malignant transformation. The patient had undergone craniotomy and tumor removal 7 years previously. Annual follow-up MRIs had shown evidence of slow tumor recurrence. With the sudden onset of seizure, the patient was diagnosed as hemorrhagic recurrence and underwent second tumor removal highly suspecting malignant change into higher grade glioma. Histopathology confirmed diffuse astrocytoma without malignant changes. As the patient's postoperative condition was excellent, we plan to withhold chemotherapy and radiation therapy for use as a later treatment option.
Malignant transformation is a common prognosis in the progression of low grade glioma (LGG) [1,2]. In the literature, recurrence of LGG into higher grade histology is seen in up to 13% to 86% of cases [3]. The incidence of bleeding in all types of glioma is between 3.7-7.2% and among these cases, hemorrhage in low grade glioma counts for less than 1% [1,2]. LGG is a slowly-progressing tumor with various clinical complications [4]. Intracranial tumor bleeding is a rare clinical presentation, and a hemorrhagic event in LGG mostly indicates malignant transformation of the disease. We reviewed the published literature of hemorrhagic recurrence of low grade glioma and report a case of diffuse astrocytoma with hemorrhagic recurrence 7 years after initial diagnosis without malignant transformation.
A 23-year-old female was admitted with computed tomography (CT) and magnetic resonance image (MRI) findings of intracranial tumor bleeding, after presenting with clinical symptom of dysarthria a week before admission. The patient had a past medical history of seizure due to diffuse astrocytoma, diagnosed 7 years previously. Initially she had undergone craniotomy and total tumor removal at another institution. The pathologic result from the first operation was diffuse astrocytoma, World Health Organization (WHO) grade II and afterwards the patient had not undergone chemotherapy or radiation therapy. Her annual follow-up MRI showed slow changes in most medial corner of the tumor margin in MR fluid attenuation inversion recovery (FLAIR) image which was not recognized until they were review retrospectively (Fig. 1). She was free of symptom for 7 years until she developed seizure with dysarthria and mild headache. The patient immediately underwent CT scanning which revealed hemorrhage at the right temporal lobe correlating with her tumor site. She was admitted to our hospital a week after the initial onset of the symptoms and brain MRI scan with enhancement, diffusion, spectroscopy, and perfusion were performed to confirm the tumor site and any other changes, such as evidence of malignant transformation. On MRI imaging there was evidence of acute hemorrhage at the previous tumor site with thin rim enhancement of the mass-like lesion with surrounding edema, suggesting possible malignant change to higher grade glioma. Increased regional cerebral blood volume (about >2-2.5) from the peripheral enhancing thin rim of the Lt. perisylvian superior temporal hemorrhagic lesion was also another evidence which suggests recurred tumor with probability of malignant transformation with intratumoral recent hemorrhage (Fig. 2).
Suspecting the transformation of the hemorrhagic lesion to higher grade glioma, we performed frontotemporal craniotomy and total removal of the tumor. The tumor tissue was dissected and it was soft tissue with an irregular margin from normal brain tissue. The gross total resection of tumor was performed under navigation system.
After tumor removal, the patient recovered without any neurological deficit and is not being treated with chemotherapy or radiation therapy. We plan to withhold chemotherapy or radiation therapy for use in the event of possible later malignant recurrence. Her 4 and 16 months postoperative follow-up MRI after the second operation showed no significant differences other than post-operation changes and there was no sign or symptom of seizure or dysarthria.
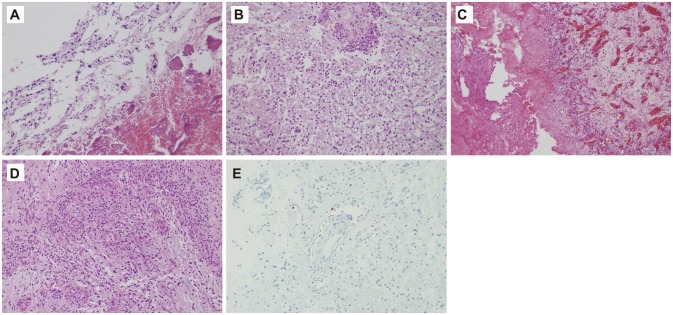
The frozen biopsy showed vascular proliferation with necrosis, which was highly suspicious of high grade giloma, with the final histopathology results revealing WHO grade II diffuse astrocytoma with red blood cells and no evidence of malignant tumor cells. The pathologist remarked that the pleomorphism of the tumor was rare and the necrotic portions were mostly hemorrhagic necrosis rather than tumor necrosis. Ki-67 showed 1-2% staining with well-differentiated astrocytoma and minimal nuclear atypia. There was no definite hypercellularity (Fig. 3). In addition, because this was her second operation, neovascularization with granular tissues along the tumor margin can be considered as postoperative changes from initial operation.
The degree of tumor malignancy is highly correlated with the bleeding tendency [5]. In general, a hemorrhagic event in low grade glioma is uncommon and to our knowledge, there has been very few case reports about hemorrhagic event or recurrence without malignant transformation after hemorrhage in other types of LGG [6,7]. White et al. [7] reported a pilocytic astrocytoma with spontaneous hemorrhage and Marton et al. [6] analyzed 37.5% malignant transformation of pleomorphic xanthoastrocytoma (PTX) to glioblastoma multiforme or anaplastic PTX in a mean period of 12.8 months, but there have been no reports of a diffuse astrocytoma, WHO grade II with hemorrhagic recurrence without malignant transformation. In our case, a series of MRI FLAIR showed slow changes in a feature on the medial corner margin of the tumor but it was not definite whether this feature was remnant tumor or recurrence at the time. There was no tumor enhancement on a follow-up MRI series and minimal changes to the surgical boundary were easy to misinterpret as postoperative changes (Fig. 1). Even if there was a remnant tumor, it is less likely to cause hemorrhage in diffuse astrocytoma without malignant change over a 7-years period with limited spread of tumor. In our patient, however, the tumor bleeding was complicated and malignant transformation was strongly suspected before the tissue biopsy. The result of biopsy showed diffuse astrocytoma WHO Grade II, indicating that the hemorrhagic event occurred without malignant transformation. A common cause of hemorrhage in patients with diffuse astrocytoma is malignant change of tumor and vascular proliferation with blood brain barrier breakdown [2]. Shibahara et al. [8] have reported the histologic finding of vascular proliferation in 25% of hemorrhagic LGG cases. In our case, however, we thought of several reasons for the hemorrhagic event. First, the proliferation of neovascularization surrounding the surgery site after the first operation could be a cause of hemorrhage. Although it is less likely to conclude that the previous operative site and postoperative changes are related to the hemorrhagic event after 7 years, it could have the potential for fragility. Second, we suspect the regrowth of tumor and possible necrotic changes could be the reason for the hemorrhage. The hemorrhage of the tumor mi-micked the original condition before the event, thus the possible necrotic changes and abnormal proliferation of tumor cannot be ruled out, because low grade astrocytoma has a tendency to transform into higher grade glioma. Della Puppa et al. [1] reported that LGG has the potential both of necrosis and abnormal vessels within the tumor, nevertheless the reasons for bleeding in low-grade gliomas are not understood.
Another consideration of this case is treatment options. Total resection of tumor is considered the treatment of choice in low grade glioma and should be followed by radiation therapy or chemotherapy unless contraindicated [9]. Treatment plans and prognosis of malignant transformed gliomas not favorable resulting in anaplastic astrocytoma or glioblastoma multifoma. The treatment of LGG is controversial because the prognosis of a progressing tumor is diverse [10,11]. It is not certain whether the natural behavior of LGG can be controlled by the benefits of radiotherapy [12]. The risks of radiotherapy may include neurocognitive impairment, radiation necrosis, brain swelling, cerebral hemorrhage or second malignancies. Therefore, there are still some major questions to be considered before beginning radiation therapy [12]. A recent prospective randomized trial from the European Organisation for Research and Treatment of Cancer reported that delaying radiation therapy until the progression of the disease does not influence overall survival, although an improvement in progression-free survival could be expected [11]. In our case, because the outcome and patient's postoperative performance was excellent, and considering the age of the patient and the benign result of the tissue confirmation, and most importantly her benign disease progression for the previous 7 years, we decided to withhold the chemotherapy and radiation therapy for later treatment options.
In conclusion, we report a rare case of hemorrhagic recurrence of diffuse astrocytoma without malignant transformation. It is extremely unusual for diffuse astrocytoma to be associated with hemorrhage without a change in characteristic of malignancy. Although favorable prognosis is expected, such an exceptional case should be closely observed and followed up for any change in the nature of the tumor and long-term course of the disease.
References
1. Della Puppa A, Zustovich F, Gardiman M, Manara R, Cecchin D, Scienza R. Haemorrhagic presentation of low-grade glioma in adults. Acta Neurochir (Wien). 2007; 149:1151–1155. discussion 1155. PMID: 17676407.

2. Rees J, Watt H, Jäger HR, et al. Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol. 2009; 72:54–64. PMID: 18632238.

3. Shafqat S, Hedley-Whyte ET, Henson JW. Age-dependent rate of anaplastic transformation in low-grade astrocytoma. Neurology. 1999; 52:867–869. PMID: 10078745.

4. Combs SE, Ahmadi R, Schulz-Ertner D, Thilmann C, Debus J. Recurrent low-grade gliomas: the role of fractionated stereotactic re-irradiation. J Neurooncol. 2005; 71:319–323. PMID: 15735924.

5. Licata B, Turazzi S. Bleeding cerebral neoplasms with symptomatic hematoma. J Neurosurg Sci. 2003; 47:201–210. discussion 210. PMID: 14978474.
6. Marton E, Feletti A, Orvieto E, Longatti P. Malignant progression in pleomorphic xanthoastrocytoma: personal experience and review of the literature. J Neurol Sci. 2007; 252:144–153. PMID: 17189643.

7. White JB, Piepgras DG, Scheithauer BW, Parisi JE. Rate of spontaneous hemorrhage in histologically proven cases of pilocytic astrocytoma. J Neurosurg. 2008; 108:223–226. PMID: 18240915.

8. Shibahara I, Kanamori M, Kumabe T, et al. Hemorrhagic onset of pilocytic astrocytoma and pilomyxoid astrocytoma. Brain Tumor Pathol. 2009; 26:1–5. PMID: 19408090.

9. Mishra KK, Puri DR, Missett BT, et al. The role of up-front radiation therapy for incompletely resected pediatric WHO grade II low-grade gliomas. Neuro Oncol. 2006; 8:166–174. PMID: 16495375.
10. Brada M, Viviers L, Abson C, et al. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003; 14:1715–1721. PMID: 14630674.

11. Karim AB, Afra D, Cornu P, et al. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys. 2002; 52:316–324. PMID: 11872276.

12. Kortmann RD, Jeremic B, Weller M, Lutterbach J, Paulsen F, Bamberg M. Immediate postoperative radiotherapy or "watch and wait" in the management of adult low-grade glioma? Strahlenther Onkol. 2004; 180:408–418. PMID: 15241528.

Fig. 1
Series of annual MRI follow-up from immediate post operation since 2003 to 2010, before the hemorrhagic event. In series of MRI fluid attenuation inversion recovery, circled lesion shows slow changes in most medial portion of the tumor boundary.
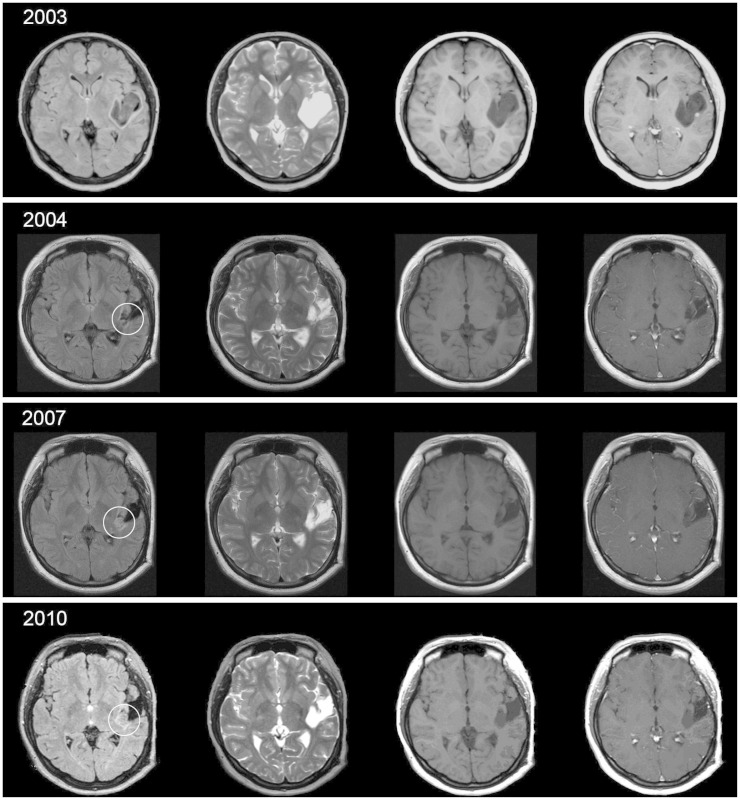
Fig. 2
Initial MRI after dysarthria and headache. A and B: The images show T1-, T2-weighted image of acute onset hemorrhage with surrounding gliotic changes from the previous operation. C and D: Flair and T1 enhancement image shows peripheral thin rim enhancing lesion about 32×28×24 mm at the left perisylvian posterior insula, subinsula, posterior external capsule white matter area, with surrounding mild edema. E and F: MR diffusion and regional cerebral blood volume (rCBV) image of the patient. Note that increase in rCBV from the peripheral enhancing thin rim of the Lt. perisylvian superior temporal hemorrhagic lesion.
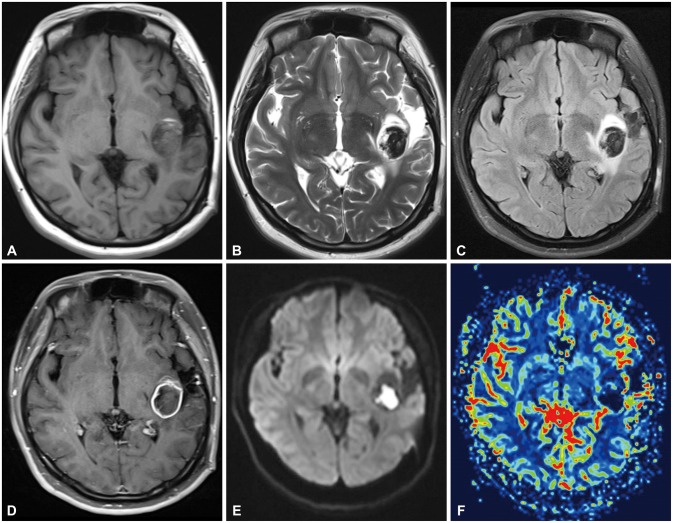
Fig. 3
Histopathology. A: Histology of 1st operation (hematoxylin-eosin stain, ×200).Tumor cells with mild nuclear atypia are present in fibrillary background, diagnosed as diffuse astrocytoma. B: Histology of main lesion of 2nd operation (hematoxylin-eosin stain, ×200). The neoplastic astrocytes with occasional nuclear atypia and glomeruloid vessels are present in the background of a loosely structured or microcystic tumor matrix, diagnosed as astrocytoma. C: Main lesion 2nd operation (hematoxylin-eosin stain, ×100). Evidence of hemorrhage and vascular proliferation of the main lesion is present. D: Main lesion 2nd operation (hematoxylin-eosin stain, ×200). Neovascularization is identified along the edge of tumor periphery. E: There is 1-2% stain of tumor cells in Ki-67 immunohistochemical staining (×200).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download