Abstract
Acute lymphoblastic leukemia (ALL) is the most common form of childhood cancer and may exhibit central nervous system (CNS) involvement. Advances in chemotherapy and effective CNS prophylaxis have significantly decreased the incidence of CNS relapse of ALL to 5-10%. Here, we report the case of a patient with isolated CNS relapse of standard risk group pre-B-cell type ALL in an 11-year-old girl, relapsed 3 years after successful completion of chemotherapy. An 11-year-old girl visited our hospital complaining of headache, dizziness, vomiting, and visual field defects. Neurological examination revealed left-side homonymous hemianopsia. Brain magnetic resonance imaging showed a large irregular dural-based sulcal hematoma in the right parietal and occipital lobes. Surgery to remove the hematoma revealed the existence of hematopoietic malignancy after pathologic evaluation. Bone marrow biopsy was subsequently performed but showed no evidence of malignancy.
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children, with complete remission (CR) rates reaching 70-80%. It accounts for approximately 25% of all childhood cancers and almost 75% of childhood leukemias [1]. CR is defined as the presence of less than 5% leukemic blast cells in bone marrow, absence of tumor cells from peripheral blood, and absence of other sign and symptoms of the disease. As leukemia cells sometimes invade the central nervous system (CNS), most treatment protocols include chemotherapy delivery into the CNS fluid, termed intrathecal chemotherapy. Remarkable advances in therapeutic regimens and the commencement of CNS-directed treatment have reduced the risk of CNS relapse for childhood ALL to 5-10% [2].
Here, we report our experience of a patient with isolated CNS relapse of standard risk group pre-B-cell type ALL in an 11-year-old girl, relapsed 3 years after successful completion of chemotherapy (i.e., remission induction, consolidation, reintensification, and maintenance therapy for 2 years).
An 11-year-old girl came to our outpatient department in February 2011 with symptoms of headache, dizziness, vomiting, and visual field defects that had begun 2 days earlier. Neurologic examination on admission showed sleeping tendency and left-side homonymous hemianopsia.
She had a history of ALL that was found as a result of an asymptomatic enlarged neck and axillar lymph node mass in June 2006. Needle aspiration and bone marrow biopsy were performed, followed by peripheral blood study. In bone marrow biopsies, cells are positive for CD10 (11%), CD19 (28%), CD22 (24%) and negative for T-cell lymphoid markers and myeloid markers such as CD3, CD5, CD7, CD33. Chromosomal study was not available due to scanty mononuclear cell count (<500/µL). In cerebrospinal fluid study, no evidence of malignant was found. She was diagnosed as having standard-risk pre-B-cell ALL. As the CCG 1952A regimen, four weeks of induction chemotherapy (prednisone+vincristine+L-asparaginase+intrathecal-cytosine arabinoside+intrathecal-methotrexate), four weeks of consolidation chemotherapy (prednisone+vincristine+6-mercaptopurine+intrathecal-methotrexate), eight weeks of interim maintenance (vincristine+prednisone+methotrexate+6-mercaptopurine), eight weeks of delayed-intensification chemotherapy (dexamethasone+vi-ncristine+doxorubicin+L-asparaginase+cyclophosphamide+ 6-thioguanine+cytosine arabinoside+intrathecal-methotrexate), and repeated second cycle of interim maintenance, delayed-intensification. Subsequently, two years of maintenance chemotherapy (prednisone+vincristine+6-mercaptopurine+methotrexate+intrathecal-methotrexate) were administered until August 2008. After the standard therapy, she had no evidence of disease for 3 years.
Non-enhanced computed tomography of the brain revealed a large irregular dural-based high-density lesion; it seemed to be a sulcal hematoma in the right parieto-occipital lobe (Fig. 1).
Brain magnetic resonance imaging demonstrated a low-intensity lesion on both T1- and T2-weighted images that appeared to be a sulcal hematoma. Gadolinium-enhanced T1-weighted imaging showed a heterogeneously enhanced dural-based mass-like lesion and sulcal enhancement (Fig. 2).
Right occipital craniotomy, tumorectomy, and duroplasty were performed. When the bone flap was removed, we found suspicious invasive bony lesions on the inner cortex of the skull and dura mater. After dural incision, the hematoma was thrust out. Regarding gross findings, the hematoma had a mass-like appearance and adhered to cerebral vessels; it appeared to be a hematologic malignancy. Pathologic analysis revealed hematologic malignant cells in frozen sections.
The dark brown-colored tumor and gliotic brain parenchyma had ambiguous margins. Furthermore, infiltration of the superior sagittal sinus was suspected. The tumor was meticulously excised without vascular injury or bleeding control. Some residual mass on the superior sagittal sinus was cauterized. Duroplasty and bone flap fixation were subsequently performed.
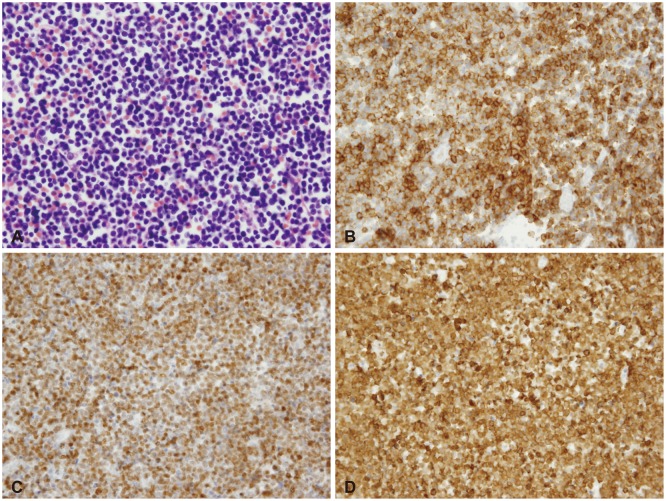
Pathological examination showed the neoplasm was composed of hematopoietic malignant cells (Fig. 3A); they were found to be B-cell lymphoblastic leukemia as they expressed lymphoblast markers [e.g., Terminal deoxynucleotidyl transferase (Fig. 3C) and CD10 (Fig. 3B)] and B-cell lymphoid marker (e.g., CD79a) (Fig. 3D) with moderate intensity. No myeloid markers or T-cell lymphoid markers (e.g., CD3) were expressed.
The patient fully recovered without any complications. To monitor leukemia recurrence, additional laboratory tests including peripheral blood smear, chromosomal study, and bone marrow biopsy were performed. However, there was no subsequent evidence of malignancy. Thus, the cerebral lesion was the only manifestation of disease. For adequate therapy, she was transferred to the Department of Pediatric Oncology of Seoul National University Hospital. According to her medical records, she received reinduction chemotherapy by means of CCG-1884 protocol, and craniospinal irradiation (24 Gy to brain, 6 Gy to spinal). And then, she received peripheral blood stem cell transplantation.
Combination chemotherapy consisting of steroid and vincristine was developed in the 1950s. This was supplemented by effective CNS preventive therapy in the 1960s, resulting in great advances in treatment. The evolution of multidrug chemotherapy enhances patients' long-term event-free survival (EFS) rate, which is currently above 75%. Despite the current cure rate, relapse remains the most pressing obstacle. Relapse in the CNS also can occur alone or with accompanying bone marrow infiltration. Most of ALL relapses occur during treatment or within the first 2 year after treatment completion, although relapses have been reported to occur even after 10 years from diagnosis.
Central nervous system-directed treatment is a significant contributing factor for improving the survival rate of children with ALL. It is an essential part of treatment that not only reduces the rate of CNS relapse, but the incidence of bone marrow recurrence as well. Although less than 5% of patients with ALL actually present with overt CNS leukemia, without prophylactic CNS-directed treatment, over 50% will develop CNS disease [3]. Presenting features associated with an increased risk of CNS relapse in pediatric patients include a T-cell immunophenotype, hyperleukocytosis, high-risk genetic abnormalities such as the Philadelphia chromosome and t(4;11), and the presence of leukemic cells in cerebrospinal fluid (even from iatrogenic introduction due to a traumatic lumbar puncture). More recently, polymorphisms in genes that code for proteins involved in the pharmacodynamics of antileukemic drugs have been associated with the risk of CNS relapse [4].
The most commonly used method of CNS-directed treatment in the 1970s and 1980s consisted of cranial irradiation (originally a dose of 24 Gy, later reduced to 18 Gy) and intrathecal chemotherapy with methotrexate. However, cranial radiation is associated with various forms of damage to normal brain tissue, including leukoencephalopathy, mineralizing microangiopathy, and the development of secondary tumors [5]. Because such damage can induce long-term neurocognitive impairments, therapeutic regimens have been modified to reduce or eliminate cranial radiation, substituting it with intrathecal and systemic chemotherapy [6].
A large retrospective analysis of CNS relapse recently reported no difference in relapse rates between patients who received and did not receive cranial irradiation. Pui et al. [7] demonstrate that with effective risk-adjusted systemic chemotherapy and intrathecal therapy initiated at diagnosis, cranial irradiation can safely be omitted from CNS-directed treatment without compromising overall survival in all newly diagnosed ALL patients.
Central nervous system relapse rate can be reduced by introducing specific CNS-directed prophylaxis earlier, substituting dexamethasone for prednisone, which is associated with lower CNS and systemic relapse rates in pediatric and adult ALL patients [8], and by using CNS irradiation only for patients at high risk of CNS relapse, such as patients with T-cell ALL who have high initial white blood cell counts [9].
The CNS recurrence rate is low with the use of current protocols. However, until recently, there is no define therapy for patients who experienced CNS relapse and had poor outcomes. For example, the overall 5-year survival rates of patients with CNS relapse within 18 and 36 months of initial diagnosis were only 43% and 68%, respectively [10].
An improved outcome for these patients was recently reported by Pediatric Oncology Group (POG) study 9061 (71% of 4-year EFS). It is consisted with 6-month chemotherapy with drugs known to have effective CNS penetration and craniospinal irradiation. However, failures still occurred in the bone marrow. In addition, craniospinal radiation exposed patients to potential risks of long-term neurocognitive deficits, and secondary brain tumors. In POG study 9,061, duration of initial remission was an important prognostic factor for children with isolated CNS leukemia, resulting in 4-year EFS for patients with durations of at least 18 months of 83% versus 46% for those with less than 18 months (p=0.0002) [11].
There is POG's more recent regimen for isolated CNS relapse of ALL, POG 9,412. It is consisted with 6 additional months of intensive systemic chemotherapy. Barredo et al. [12] reported that 6 additional months of intensive systemic chemotherapy would improve survival by decreasing subsequent systemic relapses while still eradicating meningeal leukemia. Furthermore, reduced cranial radiation (18 Gy) without spinal radiation could be the treatment of choice for ALL patient with isolated CNS relapse and duration of first CR at least 18 months.
The strategy of delaying cranial or craniospinal irradiation for 6 to 12 months to allow initial intensification of systemic chemotherapy has yielded long-term second EFS rates of 70% to 80% in children with isolated CNS relapse [4]. Nonetheless, CNS relapse in pediatric patients with a short initial remission duration, T-cell ALL, or prior cranial irradiation continues to pose a therapeutic challenge.
Future direction of treatment strategies that could improve outcome in patients who have had a CNS relapse include frequent
and early intrathecal therapy which can maintain a therapeutic level of cytarabine in cerebrospinal fluid for 2 weeks or more. Ongoing studies are testing whether the dose of cranial irradiation can be further reduced in patients with isolated CNS relapse and long initial remissions. In the Rotterdam-84 CNS-ALL chemotherapy protocol, intensive systemic and intraventricular therapy (methotrexate 6 mg on days 1 and 3, and cytarabine 60 mg on day 2) without the use of CNS radiotherapy yielded a 5-year EFS rate of 59±14% (standard error) among 13 children with isolated CNS relapse who had no prior irradiation [4].
In conclusion, we reported the case of a patient with isolated CNS relapse of standard risk group pre-B-cell type ALL in an 11-year-old girl, relapsed 3 year after successful completion of chemotherapy. Leukemic meningitis is common presentation of CNS involvement. But, as mentioned above, parenchymal hemorrhage due to vascular slugging of leukocyte can be presentation of CNS involvement.
References
1. Badr MA, Hassan TH, El-Gerby KM, Lamey ME. Magnetic resonance imaging of the brain in survivors of childhood acute lymphoblastic leukemia. Oncol Lett. 2013; 5:621–626. PMID: 23420690.

2. Schroeder H, Garwicz S, Kristinsson J, Siimes MA, Wesenberg F, Gustafsson G. Outcome after first relapse in children with acute lymphoblastic leukemia: a population-based study of 315 patients from the Nordic Society of Pediatric Hematology and Oncology (NOPHO). Med Pediatr Oncol. 1995; 25:372–378. PMID: 7674994.

3. Barredo J, Ritchey AK. Controversies in the management of central nervous system leukemia. Pediatr Hematol Oncol. 2010; 27:329–332. PMID: 20469977.

4. Pui CH. Central nervous system disease in acute lymphoblastic leukemia: prophylaxis and treatment. Hematology Am Soc Hematol Educ Program. 2006; 142–146. PMID: 17124053.

5. Chan MS, Roebuck DJ, Yuen MP, Li CK, Chan YL. MR imaging of the brain in patients cured of acute lymphoblastic leukemia--the value of gradient echo imaging. AJNR Am J Neuroradiol. 2006; 27:548–552. PMID: 16551991.
6. Gay CT, Bodensteiner JB, Nitschke R, Sexauer C, Wilson D. Reversible treatment-related leukoencephalopathy. J Child Neurol. 1989; 4:208–213. PMID: 2768785.

7. Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009; 360:2730–2741. PMID: 19553647.

8. Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005; 129:734–745. PMID: 15952999.

9. Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008; 111:2548–2555. PMID: 18039957.
10. Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Childrens Oncology Group study. Leukemia. 2008; 22:2142–2150. PMID: 18818707.

11. Ritchey AK, Pollock BH, Lauer SJ, Andejeski Y, Barredo J, Buchanan GR. Improved survival of children with isolated CNS relapse of acute lymphoblastic leukemia: a pediatric oncology group study. J Clin Oncol. 1999; 17:3745–3752. PMID: 10577846.

12. Barredo JC, Devidas M, Lauer SJ, et al. Isolated CNS relapse of acute lymphoblastic leukemia treated with intensive systemic chemotherapy and delayed CNS radiation: a pediatric oncology group study. J Clin Oncol. 2006; 24:3142–3149. PMID: 16809737.

Fig. 1
Computed tomography of the brain revealed a large irregular dural-based high-density lesion in right parieto-occipital lobe.
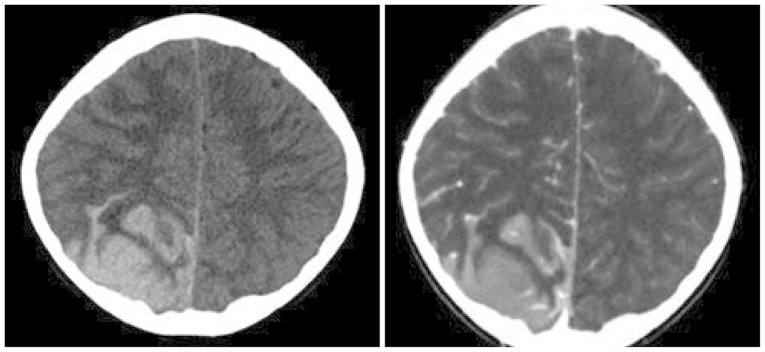
Fig. 2
Brain magnetic resonance imaging demonstrated a low-intensity lesion on both T1- (A) and T2- (B) weighted images that appeared to be a sulcal hematoma. Gadolinium-enhanced T1-weighted imaging (C) showed a heterogeneously enhanced dural-based mass-like lesion and sulcal enhancement.
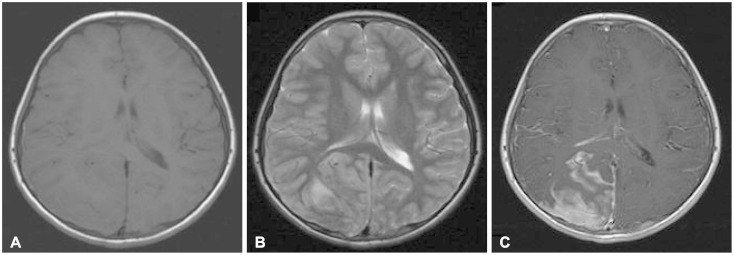
Fig. 3
Photomicrographs of the tumor specimen. A: H&E stain (×600). Several lymphoblasts with high nuclear/cytoplasmic ratio and variably condensed nuclear chromatin. B: Immunophenotype stain (CD10). Lymphoblast marker (CD10) was positive. C: Immunophenotype stain (TdT). Lymphoblast marker (TdT) was positive. D: Immunophenotype stain (CD79a). B-cell marker (CD79a) was positive.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download