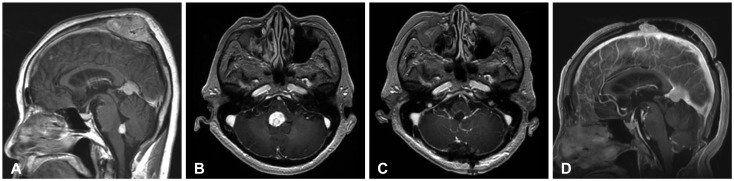
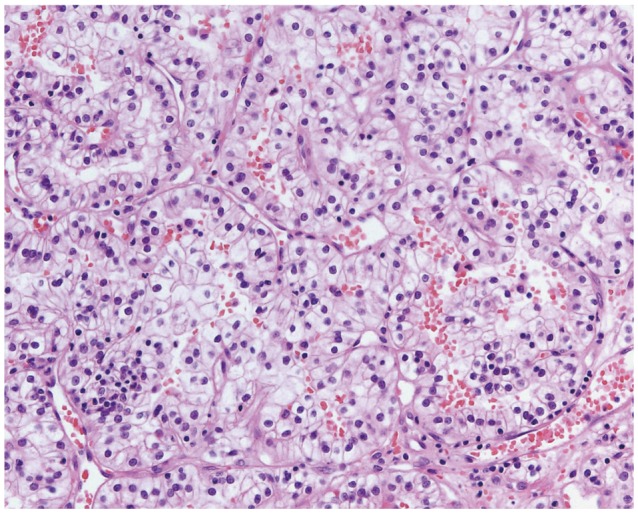
1. Kim YH, Kim JW, Chung HT, Paek SH, Kim DG, Jung HW. Brain metastasis from renal cell carcinoma. Prog Neurol Surg. 2012; 25:163–175. PMID:
22236678.

2. Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery in patients with renal cell carcinoma metastasis to the brain: long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg. 2003; 98:342–349. PMID:
12593621.

3. Miyao N, Naito S, Ozono S, et al. Late recurrence of renal cell carcinoma: retrospective and collaborative study of the Japanese Society of Renal Cancer. Urology. 2011; 77:379–384. PMID:
20970828.

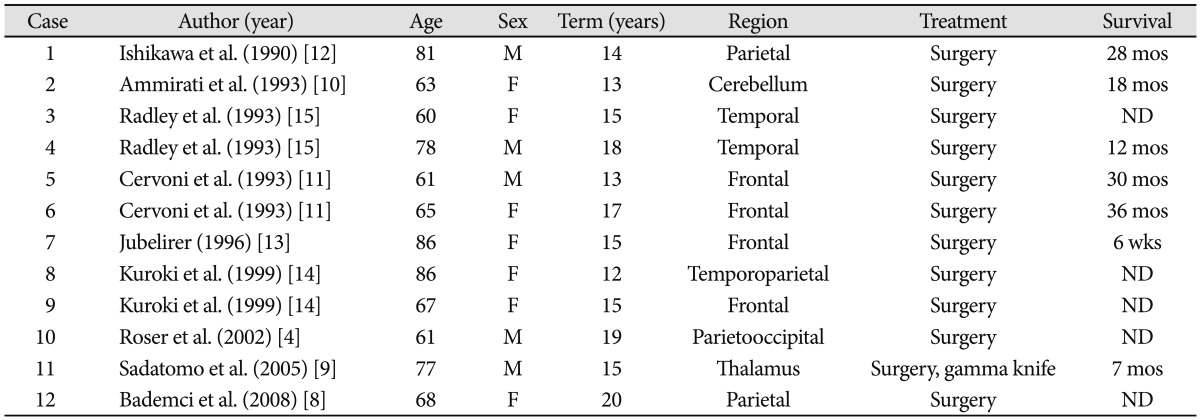
4. Roser F, Rosahl SK, Samii M. Single cerebral metastasis 3 and 19 years after primary renal cell carcinoma: case report and review of the literature. J Neurol Neurosurg Psychiatry. 2002; 72:257–258. PMID:
11796778.

5. Shuto T, Matsunaga S, Suenaga J, Inomori S, Fujino H. Treatment strategy for metastatic brain tumors from renal cell carcinoma: selection of gamma knife surgery or craniotomy for control of growth and peritumoral edema. J Neurooncol. 2010; 98:169–175. PMID:
20405309.

6. Shuch B, La Rochelle JC, Klatte T, et al. Brain metastasis from renal cell carcinoma: presentation, recurrence, and survival. Cancer. 2008; 113:1641–1648. PMID:
18671240.
7. Tapper H, Klein H, Rubenstein W, Intriere L, Choi Y, Kazam E. Recurrent renal cell carcinoma after 45 years. Clin Imaging. 1997; 21:273–275. PMID:
9215475.

8. Bademci G, Bozdogan O, Berdan F, Evliyaoglu C. Extremely delayed renal cell carcinoma metastasis mimicking convexity meningioma. Neurocirugia (Astur). 2008; 19:562–564. PMID:
19112550.

9. Sadatomo T, Yuki K, Migita K, Taniguchi E, Kodama Y, Kurisu K. Solitary brain metastasis from renal cell carcinoma 15 years after nephrectomy: case report. Neurol Med Chir (Tokyo). 2005; 45:423–427. PMID:
16127263.
10. Ammirati M, Samii M, Skaf G, Sephernia A. Solitary brain metastasis 13 years after removal of renal adenocarcinoma. J Neurooncol. 1993; 15:87–90. PMID:
8455067.

11. Cervoni L, Salvati M, Delfini R. Late solitary cerebral metastasis from renal carcinoma. J Neurosurg Sci. 1993; 37:247–249. PMID:
7931650.
12. Ishikawa J, Umezu K, Yamashita H, Maeda S. Solitary brain metastasis from renal cell carcinoma 14 years after nephrectomy: a case report. Hinyokika Kiyo. 1990; 36:1439–1441. PMID:
2075881.
13. Jubelirer SJ. Late solitary cerebral metastasis from renal cell carcinoma: a case report and review of the literature. W V Med J. 1996; 92:26–27. PMID:
8599244.
14. Kuroki K, Taguchi H, Sumida M, Daimaru Y, Onda J. [Cerebral metastasis from a renal cell carcinoma more than 10 years after nephrectomy: report of two cases]. No Shinkei Geka. 1999; 27:89–93. PMID:
10024991.
15. Radley MG, McDonald JV, Pilcher WH, Wilbur DC. Late solitary cerebral metastases from renal cell carcinoma: report of two cases. Surg Neurol. 1993; 39:230–234. PMID:
8456388.

16. Clarke JW, Register S, McGregor JM, et al. Stereotactic radiosurgery with or without whole brain radiotherapy for patients with a single radioresistant brain metastasis. Am J Clin Oncol. 2010; 33:70–74. PMID:
19652578.

17. Kanner AA, Bokstein F, Blumenthal DT, Ram Z. Surgical therapies in brain metastasis. Semin Oncol. 2007; 34:197–205. PMID:
17560981.

18. Caffo M, Barresi V, Caruso G, et al. Innovative therapeutic strategies in the treatment of brain metastases. Int J Mol Sci. 2013; 14:2135–2174. PMID:
23340652.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download