Abstract
Primary intracranial fibrosarcomas (PIFs) are extremely rare and the origin of these tumors is still controversial. The rarity of primary intracranial fibrosarcomas makes it difficult to diagnose them correctly and establish a standard treatment. The pathologic diagnosis is made by distinguishing findings from light microscopic and immunohistochemistry analysis. PIFs have been known to be very aggressive neoplasms. The extra-axial location of the tumor could provide an opportunity to perform a total resection even if it does not mean a cure. We present a case of PIFs mimicking a falx meningioma in a 17-year-old man.
Primary intracranial fibrosarcomas (PIFs) are very rare and aggressive tumors. Only 41 cases have been reported in the literature to date [1,2]. Intracranial sarcomas are found in patients of all ages, although a greater number of young patients were reported in earlier studies [3]. It is thought that primary meningeal sarcoma originates from fibroblastic elements within the brain parenchyma or its meningeal coverings. Tumor bleeding is an uncommon event of this tumor [4]. The common location of PIFs is supratentorial and superficial, adherent to the dura mater.
In this report, we report a rare case of PIF mimicking a falx meningioma presenting with intratumoral hemorrhage in an adolescent.
A 17-year-old man presented to our hospital with an episode of convulsions. The patient complained of a three-month history of headache before visiting a hospital. A papilledema with decreased visual acuity was detected on neurological examination. Computed tomography revealed a large extra-axial mass with hemorrhage occupying both frontal areas (Fig. 1A). Magnetic resonance images showed a large mass with strong enhancement and T2 iso-signal intensity in both frontal lobes abutting against the falx cerebri (Fig. 1B-D). Both distal anterior cerebral arteries (ACAs) were encapsulated by the tumor. Trans-femoral cerebral angiography revealed that both ACAs were the main tumor-feeding arteries and both ACAs had multiple aneurysms, which may be aberrantly developed from increased blood flow (Fig. 1E, F).
The patient underwent surgical tumor resection after a presumed diagnosis of falx meningioma or hemangiopericytoma. Profuse bleeding from the hypervascular tumor made it difficult to resect the tumor at one stage. The tumor was well-demarcated and quite soft in a superficial portion but it was very hard in the deeper area (Fig. 2). The second and third operations were performed at 10 days and 17 days after the first surgery, respectively. The aneurysm located at the A2-3 junction was ruptured in the middle of the surgery but clipping the neck of the aneurysm was safely performed without compromising the ACA. Maximal safe resection was performed through three serial operations and only a small part of the tumor, which was tightly adhered to the ACA, remained (Fig. 3A-C). After the surgery, neurological examinations revealed a left side hemiparesis.
Histologically, the tumor based on the dura (Fig. 4A) was composed of a high cellular proliferation arranged in sheets occasionally showing a herringbone and fascicular pattern alternating (Fig. 4B) with an abundant extracellular collagenous matrix formation (Fig. 4C). No whorling of tumor cells or psammoma bodies were present. And malignant small round cell features were seen focally (Fig. 4D). High mitotic activity was noted up to 25 per 10 high power fields. Differential diagnosis included malignant spindle cell tumors including malignant hemangiopericytoma, malignant solitary fibrous tumor, monomorphic synovial sarcoma, Ewing's sarcoma and fibrosarcoma. Immunostains including CD34, CD68, S-100, smooth-muscle actin, desmin, myoglobin, CD99, epithelial membrane antigen, glial fibrillary acidic protein, CD56, mixed cytokeratin and INI-1 were performed. The tumor cells showed strong vimentin immunoreactivity and no loss of INI-1 immunoreactivity, and were negative for the other immunofluorescence stainings including epithelial membrane antigen (EMA), CD99 and CD34. Fusion gene transcripts including EWS-EWS-FLI1 t (11;22) specific for Ewing's sarcoma and SYT/SSX t (X;18) specific for synovial sarcoma were not observed by reverse transcription-polymerase reaction. These histologic, immunohistochemical and molecular findings made a confirmatory diagnosis of the fibrosarcoma and excluded the other possibilities of sarcomas in the tumor of the patient. Intracranial metastasis of systemic sarcoma was ruled out by positron emission tomography computed tomography (PET-CT) (Fig. 3D). He received gamma knife radiosurgery for the residual mass at 14 days after the last surgery. At that time, the tumor size was 1.3 cc, and he received a dose of 18 Gy, which was prescribed to the 55% isodose line.
The rarity of PIFs makes it difficult to diagnose correctly. Primary intracranial sarcomas account for 0.5-2.7% of intracranial neoplasms [5,6]. However, in up-to-date papers, the incidence of fibrosarcoma was reported in only one of 25,000 cases. The reason for the decrement in the incidence is thought to be due to advances in diagnostic techniques, resulting in previously misdiagnosed fibrosarcomas being diagnosed as other tumors such as malignant fibrous histiocytomas [3]. PIFs occur in all ages but are more prevalent in younger ages and are known to have a poor prognosis. Spontaneous hemorrhage from PIFs has been reported in a few cases but the incidence is not clearly documented to date [4]. PIFs may be induced secondarily by radiation and immunosuppressive therapy. Because PIFs are thought to arise from the mesenchymal cells in duramater, leptomeninges, vascular adventitia, the stalk of the choroid plexus or tela choroidea, the origin of PIFs is still under debate [7]. A differential diagnosis is needed with malignant meningioma, hemangiompericytoma, gliosarcoma, Ewing's sarcoma, and so on.
Pathologically, fibrosacroma of the brain is a rare primary malignancy and shares the same histologic features at any other body site. It is mandatory to exclude other malignant spindle cell tumors to make a final diagnosis of fibrosarcoma. Clinical impression of a meningioma in this patient was easily ruled out due to a lack of whorling of tumor cells or psammoma bodies and EMA immunonegativity. No staghorn-branching vascular pattern, immunonegativity of CD34 and CD99, and no fusion gene transcripts were able to exclude a possibility of major differential diagnoses including malignant hemangiopericytoma, malignant solitary fibrous tumor, monomorphic synovial sarcoma, or Ewing's sarcoma. A classical herring-bone arrangement of the tumor cells and abundant extracellular collagenous matrix primarily suggested a fibrosarcoma rather than other spindle cell malignancies [1,8]. All these findings confirmed the diagnosis of a primary fibrosarcoma of the brain based on the leptomeninges.
There is no standard treatment for primary intracranial fibrosarcoma due to its rarity and poor prognosis. In our case, three serial operations were performed for maximal safe resection. The remaining tumor was treated by radiosurgery and adjuvant chemotherapy. In the absence of established treatment, complete or maximal safe resection could provide the opportunity for longer survival to the patient.
Figures and Tables
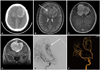 | Fig. 1Large mass with hemorrhage originating from the falx cerebi (A: Non-enhanced CT, B: T2-weighted MR), ACA was encapsulated by the tumor (white arrow). Well-enhancing tumor with hemorrhage and suspicious brain parenchyma invasion on gadolinium enhancement (C: Axial view, D: Coronal view). Large hypervascular mass in both frontal areas fed by pial branches of both ACAs. Multiple aneurysm, ecstatic changes and luminal irregularities in the A2-3 junction (E: Angiography, F: Angiography 3D). CT: computed tomography, MR: magnetic resonance, ACA: anterior cerebral artery, 3D: three dimension. |
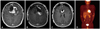 | Fig. 3Images of MRI and PET-CT. A: Residual tumor in the bifrontal region (1st stage post-operation). B: Further resection of residual tumor (2nd stage post-operation). C: Small residual tumor in the anterior pericallosal region (3rd stage post-operation). D: No significant abnormal hypermetabolic lesion suggesting malignancy except brain. PET-CT: positron emission tomography-CT. |
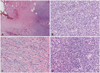 | Fig. 4Histologic features of tumor. A: Tumor based on the dura (H&E, ×40). B: Malignant spindle cell proliferation with herringbone and fascicular pattern (H&E, ×200). C: Tumor with abundant extracellular collagenous matrix production (H&E, ×200). D: Focal presence of malignant small round cell features (H&E, ×200). |
References
2. Torres G, Petit F, Vilchez V, et al. Primary cerebral fibrosarcoma in a child. Clin Neuropathol. 2007; 26:284–287.

3. Bisogno G, Roganovic J, Carli M, et al. Primary intracranial fibrosarcoma. Childs Nerv Syst. 2002; 18:648–651.

4. McDonald P, Guha A, Provias J. Primary intracranial fibrosarcoma with intratumoral hemorrhage: neuropathological diagnosis with review of the literature. J Neurooncol. 1997; 35:133–139.
5. Vatsal DK, Sharma S, Renjen PN, Kaul S, Jha AN. Primary fibrosarcoma of brain. Neurol India. 2000; 48:396–398.
6. Paulus W, Slowik F, Jellinger K. Primary intracranial sarcomas: histopathological features of 19 cases. Histopathology. 1991; 18:395–402.

7. Donnet A, Figarella-Branger D, Grisoli F. Primary meningeal fibrosarcoma: a particular neuroradiological presentation. J Neurooncol. 1999; 42:79–83.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download