Abstract
Objective
The objective of this study was to describe and characterize the clinical course of treatment for invasive prolactinoma patients using bromocriptine.
Methods
The study group included 23 patients who were treated with bromocriptine for their invasive prolactinomas. Clinical histories, serum prolactin level and pituitary hormone assessments, tumor diameter and signal intensity on sella magnetic resonance imaging (MRI), visual field exams and the dosage of medications were reviewed for each patient.
Results
During 30 months (median, range 6-99) of follow-up period, 19 patients treated with bromocriptine alone achieved biochemical remission. Four patients changed the medication to cabergoline due to the adverse effects or observed resistance of bromocriptine treatment. All of five patients who had visual symptoms improved after the course of medication. Four surgically treated patients were not able to discontinue medication because they could not maintain biochemical remission state without medication. Multivariate analysis showed that decreased enhancement on the initial followed MRI after medication and longer follow-up periods were associated with higher radiologic response.
Conclusion
We reassure that the dopamine agonist is safe and effective for the treatment of invasive pituitary adenomas. Meanwhile, surgery has a limited role on biochemical remission. Decreased enhancement on the initial follow-up MRI after medication may reflect the treatment response. Further study is required to validate the role of MRI or other factors on the actual prognosis.
Prolactin secreting adenomas account for approximately 50 to 60% of all functional pituitary tumors. Invasive prolactinomas, an extreme subset of prolactinoma, is often characterized by its extreme size, high aggressiveness, massive extrasellar involvement, and very high plasma prolactin levels. Although a microprolactinoma is found predominantly in young female patients, an invasive prolactinoma is prevalent in young male patients [1]. Patients with invasive prolactinoma usually present with symptoms or signs caused by the compression of surrounding structures by large or invasive tumors, including headache, visual disturbance, and/or diplopia which needs prompt management [2]. Many patients also have symptoms or signs of hypopituitarism, including hypogonadism [3].
Although transsphenoidal microsurgery has been applied in many cases of invasive pituitary tumors, complete surgical removal of invasive prolactinomas is almost impossible due to the high potential for invasion into surrounding structures, including the cavernous sinus [4]. Therefore, the persistence and recurrence of invasive prolactinomas following surgical procedures are very high [5,6]. Biochemical remission is also rare even after extensive tumor removal and therefore, medical treatment using bromocriptine (BROC) or cabergoline is the mainstay for treating invasive prolactinomas [7,8]. However, there were only few long term data regarding the treatment outcomes of invasive prolactinomas, which may due to the rarity of cases.
Therefore, further verification of the role of BROC treatment and the characterization of the clinical course of treatment for invasive prolactinomas are necessary. We report on a group of invasive prolactinoma patients who were initially treated with BROC to describe the clinical course and to identify potential prognostic factors.
The present study included patients who were diagnosed with invasive prolactinomas at the Neurosurgery Department of the Samsung Medical Center between August 2002 and December 2010 and who were initially treated with BROC. All met the criteria for invasive prolactinomas: 1) prolactin levels greater than 1000 ng/mL, and 2) invasion of the cavernous sinus corresponding to grade III or IV according to the classification of Knosp et al. [9]. In general, invasive giant prolactinoma is defined as larger than 4 cm in diameter. In this series, there were 8 cases which diameters were less than 4 cm but larger than 3 cm. But they fulfilled the criteria for invasiveness described above, and we defined and included these patients as invasive prolactinomas. Patients who had undergone radiotherapy or surgery before medication were excluded in this study. Clinical history, serum prolactin levels, sella magnetic resonance imaging (MRI), visual field and acuity test, and dosage of medications were reviewed in each patient.
In our study, the range of initial maximum dosage was 15-22.5 mg/day. Initial dose of medication was set by experienced endocrinologists and neurosurgeons. The maximum dose was maintained for three months to ensure a sufficient period for the drug effect even though the serum prolactin level fell below the normal range. Three to six months after the medication was started, the tumor size was measured again using sella MRI. We checked the response to treatment by evaluating the tumor size and serum prolactin levels. Medication was continued in patients who showed good response to BROC, and change to cabergoline was made if there was no significant reduction in size or prolactin level after 6 months of follow-up. Also, we considered a change in treatment medication for patients with intolerable adverse effects.
The baseline tumor size was evaluated using GE Signa 1.5 Tesla scanners (GE Medical Systems, Milwaukee, WI, USA) in the sagittal and coronal planes before and after gadolinium administration. The pituitary tumor size was evaluated from the longest diameter in the coronal or sagittal sequence of the sella MRI. Follow-up MRI examinations were repeated 3-6 months after initiation of the BROC treatment, at 12 months, and annually thereafter. The follow-up MRI protocol and analysis were similar to those used for the baseline examination. Pituitary tumor shrinkage was evaluated by the percentage reduction in the longest diameter compared with initial MRI. We also checked the signal intensity of T1-enhanced sequence. Since there was no objective standard to which one could quantify the decrease of enhancement, we checked the signal intensity ratio (region of interest defined as 2 mm2 of area which shows highest signal intensity) of pons and the mean value of three different points on each tumor on the T1 enhanced sagittal contrast enhanced (CE)-MRI image. We defined decreased signal intensity as more than a 20 percent signal intensity ratio (SIR) drop on the initial (3-6 months after treatment) follow-up MRI examination. Patients who exhibited a decreased SIR of more than 20 percent were defined as Group 1 (Fig. 1). All others were defined as Group 2 (Fig. 2).
We checked serum prolactin levels also with combined hormonal assays to evaluate the patients' pituitary insufficiency. Serum prolactin levels were measured by the ADVIA Centaur Prolactin Assay (Siemens, Malvern, PA, USA) (reference range: males, 2.1-17.7 ng/mL; females, 2.8-29.2 ng/mL). If the prolactin level was above the limitation for laboratory assay, dilutional assays were performed in all patients. In three patients, the initial prolactin level assessment was incorrect due to the use of a test protocol which had an upper limit of 1,000 ng/mL. In this study, successful biochemical remission was defined as a serum prolactin concentration of less than 30 ng/mL. Follow-up examinations took place mainly in the outpatient clinic. The three month, six month, and one year follow-up were conducted after BROC treatment; thereafter, a follow-up was undertaken every six months.
Statistical analyses were carried out using the SPSS statistical software, version 18.0 (SPSS Inc., Chicago, IL, USA). The analysis of categorical variables was performed with Fisher's exact test or chi-square test. The Mann-Whitney test was performed for continuous variables, if applicable. The independent influence of various parameters (tumor size, dosage of BROC, initial prolactin level and duration of medication) on volume reduction rate or time to biochemical remission was tested by multiple linear regression analyses. A p-value of < 0.05 was considered significant.
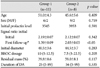
Initially 23 patients started with BROC. Seventeen male and 6 female patients were diagnosed with an invasive prolactinoma; at diagnosis, the median age was 48 years (range, 22-71). Of these 23 patients, 5 had visual field defects and 4 presented with headaches. Three patients complained of hormonal symptoms (amenorrhea, loss of libido), and one patient had a nasal obstruction and bloody rhinorrhea, which resulted from downward growing of the tumor into the sphenoid sinus. Two patients exhibited diplopia caused by limited eyeball movement. Eleven patients had no symptoms; their tumors were incidentally found during routine brain MRI or CT checkup (Table 1).
During the initial 3 months of medication, visual symptoms immediately improved in all patients (5/5). Among 2 patients with diplopia, the diplopia was improved within a month and after 3 months of medication, respectively. The headache of 4 patients was resolved within a month after BROC medication. Four of 23 patients had to change their medication because they exhibited intolerable adverse effects or biochemical resistance (Fig. 3).
When we analyzed changes in tumor size during BROC treatment, we observed a significant reduction (mean, 53%; range, 11-100%) on the latest MRI examination which was performed after 30 months (median, range 5-99) (Table 1). All patients showed a decrease in tumor size at the first follow-up MRI scan and 11 cases showed a decrease in SIR, defined as Group 1 (Fig. 1). Univariate analyses showed no difference between groups (Table 2). Multivariate analyses using the multiple linear regression test, the percentage of tumor size reduction had statistically significant correlations with duration of dopamine agonist (DA) medication (p=0.037) and the type of group (p=0.028) (Table 3), but no correlations with drug dosage (p=0.194) or initial tumor size (p=0.460).
The median follow-up period was 30 months (range, 5-99 months). Among 23 patients, 16 patients showed normal prolactin levels within 6 months of DA treatment, and 5 patients reached biochemical remission after 6 months of follow-up. Two patients did not achieve biochemical remission after 6 months of BROC medication. One patient did not achieve normal prolactin levels following 6 months of BROC medication and 5 months of carbergoline medication. The latest serum prolactin level was checked as 141.3 ng/mL. Another patient achieved biochemical remission after 2 months of cabergoline medication. The median time to achieve biochemical remission was 4 months (range 1-49 months). All patients who received surgery had their surgery after biochemical remission. But BROC were needed in all of the cases to maintain the biochemical remission state.
All patients were initially treated with BROC. Eleven patients experienced adverse side effects (Table 1). Eight out of 11 adverse effects were improved after supportive treatment. One patient's insomnia was resolved after the dose reduction (from 15 mg to 10 mg). The other two patients had to change to the cabergoline because of excessive somnolence, nausea and vomiting.
Five patients were considered to be BROC resistant. One out of 5 showed radiological resistance and other 4 biochemical resistance. One who had radiological resistance exhibited an enlarged tumor volume on the follow-up MRI. After increasing the dosage from 7.5 mg to 10 mg, the tumor volume decreased. Out of the other 4 cases that demonstrated biochemical resistance, biochemical remission could be achieved after an increase of the BROC dosage in 2 patients and by changing the medication to cabergoline in 2 patients. One patient who had changed medication to cabergoline due to BROC resistance experienced cerebrospinal fluid (CSF) leakage.
Surgical treatment was performed in 4 patients. The reasons for surgery included CSF rhinorrhea (1 patient), radiological resistance (1 patient), and localized residual tumor after BROC treatment (2 patients) (Table 4). We performed surgery for 2 patients with residual tumor for curative purposes but failed to stop medication. On postoperative follow-up MRI examinations, 3 patients exhibited small residual lesions in the cavernous sinus or parasellar portion, and 1 patient showed no demonstrable tumor. However, all patients who received surgical treatment were unable to discontinue medication to maintain biochemical remission.
Two main objectives of treatment of invasive prolactinomas are to restore normal levels of prolactin for further normalization of reproductive function, and to decompress important anatomical structures for relieving the frequently associated neurological impairment [3]. To achieve these goals, many physicians have performed a multidisciplinary approach for the treatment of invasive prolactinomas [4,10,11]. Despite these efforts, biochemical remission without DA medication seems to be difficult in most cases. Even after patients had achieved radiologic remission, they had to receive DA to maintain normal endocrine function [12,13]. The causes of increased prolactin after radiologic remission are suggested as follows: 1) prolactin secretion from residual tumors in the cavernous sinus or other areas, despite their absence in the MR images; and 2) injury to the hypothalamic-pituitary-axis in the process of the empty sella formation [12]. Considering these findings, the prolactin normalization remains an important and easily assessable end-point. Up to now, dose adjustments or medication changes have been mainly guided by attempts to obtain normalization of the hormonal levels [2,14,15]. Radiotherapy may be performed for radiologic or biochemical resistance cases. However, considering the potential side effects of this treatment such as hypopituitarism, visual deterioration, and memory impairment, the dose adjustment or changing medication should be preceded before radiotherapy [16,17]. In our case series, 19 patients (82.6%) achieved biochemical remission with BROC medication alone without significant adverse effects. Surgery was performed to discontinue or decrease DAs in three of the cases. However, none of the patients were able to discontinue the medication. Therefore, surgical procedures have only a limited role even in localized residual tumors. The following conditions are still indications for surgery: 1) occurrence of CSF leakage while taking DAs, which cannot be conservatively treated; and 2) tumor apoplexy causing significant clinical symptoms, such as sudden and severe decreases in vision [3,11]. If these indications matched and surgery is needed, determining when to perform surgery is important, because prolonged use of DAs is known to induce peritumoral fibrosis [18], which makes it more difficult to remove the tumor later and increases the risk of surgical complications. In the above surgical indication, other treatment options such as radiation therapy or medical treatment cannot replace the surgical role.
The purpose of volume reduction is to obtain decompression of important anatomical structures. In our series, compression symptoms including headache, visual acuity/field change, and limited eyeball movement were improved in all cases within 6 months of medical treatment despite the limited change in size. In the course of tumor reduction, lesions in the suprasellar area commonly shrank first, followed by those in the sphenoid sinus and the intrasellar region. Tumors in the cavernous sinus area were always the last to shrink. Most of the mass effects such as visual symptoms or headache diminished after even small decreases in volume. Some authors have reported that in cases of giant prolactinomas, visual symptoms improved several days to weeks after treatment with BROC [15,19]. Moreover, post-treatment visual improvements were not significantly different after drug administration compared to improvements observed following surgery [3,13,20].
Previous studies have demonstrated that volume decrement on initial MRI or prolonged medication may result in further volume decrement, and our findings also supported these results [12,15,19]. In addition, our results suggest that tumor contrast enhancement profiles after medication may be another predictor of the radiologic response rate. Until recently the biologic meaning of the CE-MRI parameter was unclear [21]. Neoangiogenesis of tumor and increased permeability may be assessed by measuring the value of dynamic CE-MRI. Therefore studies of the dynamic CE-MRI suggested that the degree of quantified enhancement may predict the treatment outcome of radiation therapy or hormonal therapy [22,23]. In this study, the SIR between tumor and pons were similar on the initial MRI among the patients. However, the subsequent MRI's SIR demonstrated variations, as divided into two groups which differ from decreasing SIR and unvarying SIR. The authors believed that these differences may reflect drug response or biologic activity. However, to the best of our knowledge, there has been no related study regarding invasive pituitary adenoma with respect to these issues. Therefore, further study is required to validate the role of CE-MRI on prognosis.
BROC treatment showed favorable biochemical and radiological outcomes in most patients. When treatment failure was suspected, increasing dosage of BROC or switching to carbergoline seemed to provide the best course of treatment. In the treatment of invasive prolactinomas, surgical intervention may provide only a limited role. The decreased SIR on the initial follow-up CE-MRI may be associated with the radiologic response rate. Further study is required to verify the role of CE-MRI on prognosis and clinical course of treatment.
Figures and Tables
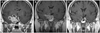 | Fig. 1Characteristic findings of decreased signal intensity ratio group patients. A: Initial magnetic resonance image (MRI). B: First follow-up MRI after 3 months of treatment showed decreased signal intensity ratio (2.27 to 1.47) on enhanced T1 image. C: Last follow-up after 48 months, MRI showed a remarkable decrease in size. |
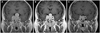 | Fig. 2Characteristic findings of constant signal intensity ratio group patients. A: Initial magnetic resonance image (MRI). B: First follow-up MRI after 4 months of treatment showed almost unchanged signal intensity ratio (2.01 to 1.98) on enhanced T1 image. C: Last follow-up after 49 months, MRI showed a decreased mass but not remarkable in size. |
References
1. Schaller B. Gender-related differences in prolactinomas. A clinicopathological study. Neuro Endocrinol Lett. 2005; 26:152–159.
2. Shimon I, Benbassat C, Hadani M. Effectiveness of long-term cabergoline treatment for giant prolactinoma: study of 12 men. Eur J Endocrinol. 2007; 156:225–231.

3. Wu ZB, Yu CJ, Su ZP, Zhuge QC, Wu JS, Zheng WM. Bromocriptine treatment of invasive giant prolactinomas involving the cavernous sinus: results of a long-term follow up. J Neurosurg. 2006; 104:54–61.

4. Fraioli MF, Novegno F, Catena E, Fraioli C, Moschettoni L. Multidisciplinary treatment of giant invasive prolactinomas in paediatric age: long-term follow-up in two children. Childs Nerv Syst. 2010; 26:1233–1237.

5. Serri O, Rasio E, Beauregard H, Hardy J, Somma M. Recurrence of hyperprolactinemia after selective transsphenoidal adenomectomy in women with prolactinoma. N Engl J Med. 1983; 309:280–283.

6. Nelson PB, Goodman M, Maroon JC, Martinez AJ, Moossy J, Robinson AG. Factors in predicting outcome from operation in patients with prolactin-secreting pituitary adenomas. Neurosurgery. 1983; 13:634–641.

7. Pinzone JJ, Katznelson L, Danila DC, Pauler DK, Miller CS, Klibanski A. Primary medical therapy of micro- and macroprolactinomas in men. J Clin Endocrinol Metab. 2000; 85:3053–3057.

8. Passos VQ, Souza JJ, Musolino NR, Bronstein MD. Long-term follow-up of prolactinomas: normoprolactinemia after bromocriptine withdrawal. J Clin Endocrinol Metab. 2002; 87:3578–3582.

9. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993; 33:610–617. discussion 617-8.

10. Molitch ME. Prolactin-secreting tumors: what's new? Expert Rev Anticancer Ther. 2006; 6:Suppl 9. S29–S35.

11. Saeki N, Nakamura M, Sunami K, Yamaura A. Surgical indication after bromocriptine therapy on giant prolactinomas: effects and limitations of the medical treatment. Endocr J. 1998; 45:529–537.

12. Wu ZB, Su ZP, Wu JS, Zheng WM, Zhuge QC, Zhong M. Five years follow-up of invasive prolactinomas with special reference to the control of cavernous sinus invasion. Pituitary. 2008; 11:63–70.

14. Delgrange E, Duprez T, Maiter D. Influence of parasellar extension of macroprolactinomas defined by magnetic resonance imaging on their responsiveness to dopamine agonist therapy. Clin Endocrinol (Oxf). 2006; 64:456–462.

15. Cho EH, Lee SA, Chung JY, et al. Efficacy and safety of cabergoline as first line treatment for invasive giant prolactinoma. J Korean Med Sci. 2009; 24:874–878.

16. McCollough WM, Marcus RB Jr, Rhoton AL Jr, Ballinger WE, Million RR. Long-term follow-up of radiotherapy for pituitary adenoma: the absence of late recurrence after greater than or equal to 4500 cGy. Int J Radiat Oncol Biol Phys. 1991; 21:607–614.

17. Turbin RE, Thompson CR, Kennerdell JS, Cockerham KP, Kupersmith MJ. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002; 109:890–899. discussion 899-900.

18. Kontogeorgos G, Horvath E, Kovacs K, et al. Morphologic changes of prolactin-producing pituitary adenomas after short treatment with dopamine agonists. Acta Neuropathol. 2006; 111:46–52.

19. Yang MS, Hong JW, Lee SK, Lee EJ, Kim SH. Clinical management and outcome of 36 invasive prolactinomas treated with dopamine agonist. J Neurooncol. 2011; 104:195–204.

20. Lesser RL, Zheutlin JD, Boghen D, Odel JG, Robbins RJ. Visual function improvement in patients with macroprolactinomas treated with bromocriptine. Am J Ophthalmol. 1990; 109:535–543.

21. Zahra MA, Tan LT, Priest AN, et al. Semiquantitative and quantitative dynamic contrast-enhanced magnetic resonance imaging measurements predict radiation response in cervix cancer. Int J Radiat Oncol Biol Phys. 2009; 74:766–773.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download