Abstract
A 55-year-old female presented to the emergency room with a complaint of aphasia. Her initial brain computed tomography scan showed an intracranial hemorrhage in the left frontal area. After surgery, histopathological examination confirmed the diagnosis of a chondroma. Intradural chondroma is a rare, slow growing, benign intracranial neoplasm, but is even rarer in combination with an intratumoral hemorrhage. Chondromas are generally avascular cartilaginous lesions. Our case was thought to be caused by the rupture of abnormally weak vessels derived from the friable tumor. Intradural chondromas may be included in the differential diagnosis of intracranial tumors with acute hemorrhages.
Intradural chondroma is a rare, slow growing, benign intracranial neoplasm, and is very occasionally observed in combination with intratumoral hemorrhage [1]. Most patients present with symptoms of increased intracranial pressure, seizures, or focal neurological deficits. Intradural chondromas usually occur as isolated lesions, but may occur alongside syndromes such as Ollier's disease or Maffuci's syndrome [2]. We report a rare case of intradural chondroma with intratumoral hemorrhage.
A 55-year-old female visited the emergency room with a complaint of aphasia. She had suffered generalized headaches for 2 months prior to presentation, and had showed memory impairment for 1 month. She had no other relevant medical history and she was right-handed. Neurological examination revealed global aphasia and right side weakness (motor grade I). Her initial brain computed tomography (CT) scan showed an intracranial hemorrhage in the left frontal area. Magnetic resonance imaging showed a 5.9×3.5 cm-sized, non-enhancing mass-like lesion with heterogeneous signal intensity in the left frontal lobe (Fig. 1). Intraoperative findings showed a friable and yellowish tumor adhered to the falx. A frozen biopsy was performed, and the tumor was tentatively diagnosed as a transitional type meningioma. The tumor with intratumoral hemorrhage was totally removed. The bleeding focus of the tumor was uncertain. After surgery, the patient's symptoms were almost totally improved and postoperative CT showed that the tumor had been completely removed (Fig. 2). Histopathological examination confirmed the diagnosis of a chondroma (Fig. 3). The lesion was isolated, not combined with other syndromes as mentioned above. The patient was discharged with only mild dysarthria. Her Karnofsky Performance Scale score was 90 at the time of discharge.
Intracranial chondromas are rare benign neoplasm, which comprise only 0.2-0.3% of all intracranial tumors [3]. Intracranial chondromas are thought to arise from ectopic hyaline cartilaginous rests trapped within suture lines [4]. Due to the rarity of intracranial chondromas, few data about this neoplasm are available. Only 3 cases were identified by H. W. Cushing in a previous series of his 2,033 cases [5].
The first patient with intracranial chondroma was reported in 1851, and the first successful surgical resection of an intracranial chondroma was reported in 1982 [6]. Two previous reviews documented 125 and 139 cases of intracranial chondromas, respectively [7,8].
The most common location of intracranial chondroma is the skull base, especially the sellar and parasellar regions [7]. The tumor may also occur in the dura mater of the convexity or the falx, as in our case. However, the exact pathogenesis of intracranial chondroma is uncertain. In the present case, it is most probable that it arose from ectopic hyaline cartilaginous rests trapped within the left coronal suture line or from the falx. Most intracranial chondromas are confined within capsules, as in this case [9].
Recently, Xin et al. [10] reviewed 30 cases of intracranial chondroma that were treated at a single institute, and none showed intratumoral hemorrhaging. Intratumoral hemorrhages often occur in malignant brain tumors such as glioblastomas and metastatic brain tumors, but chondroid tumors rarely develop intratumoral hemorrhages. Only 10 cases of hemorrhages in such tumors have been reported in detail to date, including this case [1].
Chondromas are generally avascular lesions. However, in the present case, the tumor showed intratumoral hemorrhage and a rich blood supply confined within the capsule. Linsen et al. [1] reported a case of an intracranial chondroma with intratumoral and subarachnoidal hemorrhage, and they concluded the rich blood supply was the reason for the hemorrhage. In the present case, the focus of hemorrhage was uncertain, but it was considered to be caused by the rupture of abnormally weak vessels, because intraoperative gross findings of the tumor seemed very friable.
We report a rare case of intradural chondroma with intratumoral hemorrhage. In the present case, imaging findings were not sufficient for diagnosis and the intraoperative finding did not show cartilaginous portions. Histological examination was needed to differentiate chondromas from other tumors, including cases combined with intratumoral hemorrhage. Intradural chondromas may be included in the differential diagnosis of intracranial tumors with acute hemorrhage.
Figures and Tables
Fig. 1
Preoperative MRI images show a space-occupying lesion attached to the falx and dura at the left frontal area. A: T2-weighted image. B: T1-weighted image. C: T1-weighted enhanced image. MRI: magnetic resonance imaging.
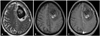
Fig. 2
Postoperative image study. A: The tumor was totally removed according to the postoperative MRI scan. B: A six month follow-up MRI scan showed no evidence of recurrence (both, T1-weighted enhanced image). MRI: magnetic resonance imaging.
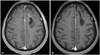
Fig. 3
Histologic finding of tumor specimen. A: An intracranial tumor, which showed a lobulated growth pattern (×40, H&E). B: Hemorrhagic areas were present inside the tumor (×100, H&E). C: Tumor cells were distributed in the myxoid cartilaginous matrix with eosinophilic cytoplasm and absence of nuclear atypism. A few tumor cells had lacunae (×400, H&E).

References
1. Linsen M, Junmei W, Liwei Z, Jianping D, Xuzhu C. An intracranial chondroma with intratumoral and subarachnoidal hemorrhage. Neurol India. 2011; 59:310–313.

2. Ghogawala Z, Moore M, Strand R, Kupsky WJ, Scott RM. Clival chondroma in a child with Ollier's disease Case report. Pediatr Neurosurg. 1991-1992; 17:53–56.
3. Colpan E, Attar A, Erekul S, Arasil E. Convexity dural chondroma: a case report and review of the literature. J Clin Neurosci. 2003; 10:106–108.

4. Padhya TA, Athavale SM, Kathju S, Sarkar S, Mehta AR. Osteochondroma of the skull base. Otolaryngol Head Neck Surg. 2007; 137:166–168.

5. Bakdash H, Alksne JF, Rand RW. Osteochondroma of the base of the skull causing an isolated oculomotor nerve paralysis. Case report emphasizing microsurgical techniques. J Neurosurg. 1969; 31:230–233.

6. Khosrovi H, Sadrolhefazi A, el-Kadi H, Bloomfield SM, Schochet SS. Intradural convexity chondroma: a case report and review of diagnostic features. W V Med J. 2000; 96:612–616.
7. Mapstone TB, Wongmongkolrit T, Roessman U, Ratcheson RA. Intradural chondroma: a case report and review of the literature. Neurosurgery. 1983; 12:111–114.

8. Matz S, Israeli Y, Shalit MN, Cohen ML. Computed tomography in intracranial supratentorial osteochondroma. J Comput Assist Tomogr. 1981; 5:109–115.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download