Abstract
We report a case of solitary primary leptomeningeal glioma. The mass was totally removed under awake surgery. Intraoperatively, no parenchymal involvement was noted. Histopathological study revealed a predominant anaplastic oligodendroglioma component and a focal anaplastic astrocytoma component, which was consistent with an anaplastic oligoastrocytoma. Adjuvant tomotherapy was followed and the tumor has not recurred until 12 months after surgery. A focal type of primary leptomeningeal glioma is extremely rare. We report a rare case of solitary primary leptomeningeal anaplastic oligoastrocytoma.
Gliomas that arise primarily in leptomeninges have rarely been reported in either cerebral hemispheres or the spinal cord [1-3]. Most reported cases have presented diffuse leptomeningeal infiltration of the tumor on their imaging studies. However, the reports of focal solitary glioma in leptomeninges are extremely rare as there have been only 16 cases reported in the literature [4]. Their differential diagnosis is very challenging because the diffuse form may mimic the clinical manifestation and radiographic appearance of chronic meningitis, while the solitary form may bear a strong histological resemblance to other central nervous system (CNS) tumors. We herein report a case of focal solitary leptomeningeal glioma which mimicked an extra-axial tumor.
A 45-year-old man presented with multiple episodes of partial seizure on his left upper and lower extremities for a year. He was previously evaluated with brain computed tomography scan when he first experienced an episode of partial seizure, which revealed a suspicious tumor on the right medial frontal lobe. As his seizure episodes became more frequent, he was evaluated with brain magnetic resonance imaging (MRI), which demonstrated a well-circumscribed extra-axial mass on the right frontal lobe. T1-weighted MRI with gadolinium showed heterogeneous subtle enhancement of the tumor. T2-weighted MRI showed a hyperintense multi-lobulated mass with minimal peri-tumoral edema and superficial siderosis on the right frontal cortex (Fig. 1).
After exposure of the tumor under awake surgery, the tumor was easily distinguished from surrounding normal brain parenchyma by gross appearance. First, motor strip was localized with an Ojemann cortical stimulator (Integra LifeSciences Corp., Plainsboro, NJ, USA). The tumor mass was easily separated from the normal brain cortex as there was no continuation between the mass lesion and normal brain parenchyma. The tumor was grayish to pinkish and very soft in consistency, and showed high vascularity. The tumor was removed completely (Fig. 2).
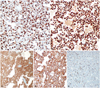
Histological examination demonstrated a relatively well-circumscribed tumor grossly. High power views showed glial tumor cells with increased cellularity and mitosis. Most of the specimen consisted of tumor cells with small round cells with perinuclear halos, but an astrocytic component was also observed focally (Fig. 3). By immunohistochemical (IHC) stains, the tumor cells were strong positive for glial fibrillary acidic protein and Olig-2. The final diagnosis was made as an anaplastic oligoastrocytoma, a glioma of World Health Organization (WHO) grade 3. IHC also showed mutation of the isocitrate dehydrogenase 1 and amplification of the epidermal growth factor receptor. Ki-67 proliferative index was about 10-15% (Fig. 4). Gene study revealed methylation of the promoter of O-6-methylguanine methyltransferase and codeletion of 1p and 19q.
Postoperatively, no neurological deficit was observed. The tumor bed was irradiated by Tomotherapy (Accuray, Madison, WI, USA) with total dose of 60 Gy and 30 times of fractionation. Brain MRI obtained at 6 and 12 months after surgery demonstrated no evidence of tumor recurrence. The patient has been on antiepileptic medication after surgery and has not experienced any episodes of seizures.
A primary leptomeningeal glioma was described by Cooper and Kernohan [5] as a "rare neoplastic condition in which glial tumor cells extend diffusely throughout the leptomeninges without forming any intra-axial lesions". As a primary leptomeningeal glioma is extremely rare, there is no consensus on the diagnostic criteria and treatment. Two anatomical and clinical forms have been described: a focal mass-forming lesion and a diffuse gliomatosis. The diffuse form constitutes the majority of this condition, in which glial tumor cells are widely disseminated outside the CNS parenchyma without mass-forming. Keith et al. [6] defined a diffuse form of leptomeningeal glioma as a primary leptomeningeal gliomatosis and reviewed 50 previously reported cases. Their diagnosis is very challenging since it only depended on imaging studies. Histopathological examination is very important because there are many conditions which should be differentiated; viral and fungal infections as well as tuberculosis and sarcoidosis. The prognosis of a diffuse primary leptomeningeal glioma is dismal and its diagnosis is often made on autopsy. On the other hand, a solitary and focal form of primary leptomeningeal glioma has been rarely reported as summarized in Table 1. They often mimic an extra-axial CNS tumor such as a meningioma [7]. Some authors defined this condition as a meningeal glioma [3]. Its prognosis is known to be better than that of a diffuse form of primary leptomeningeal glioma [8]. In our case, the tumor was apparently located outside brain parenchyma and was completely separate without continuation. The tumor did not mimic a meningioma on preoperative MRI as its enhancement on T1-weighted image was very subtle. In addition, it showed neither dural tail nor calcification. Preoperative imaging findings were not suggestive of any specific conditions such as a metastatic tumor, a glioma, as well as a meningioma.
The pathogenesis of primary leptomeningeal glioma has not been well elucidated. However, there have been several reports that heterotopic glial tissue is frequently founded in the leptomeninges [5]. Some authors proposed a possibility that this heterotopic glial cell nest is the origin of primary leptomeningeal gliomas, having been separated from normal brain parenchyma during embryogenesis and having subsequently undergone neoplastic transformation [9]. The histopathology of our case demonstrated typical findings of WHO grade 3 of glioma, and the results of immunohistochemical staining were also consistent with a glial cell tumor. The tumor mass consisted predominantly of oligodendroglioma components. It was reported that leptomeningeal glial cell nests consist predominantly of astrocytes and rarely contain oligodendrocytes and even ependymal cells [1]. It is interesting that the histopathological diagnosis of our case was an anaplastic oligoastrocytoma as most of the reported cases were diagnosed as an astrocytoma of various grades of malignancy [10].
In conclusion, although primary leptomeningeal glioma is very rare, the possibility should be taken into consideration in the differential diagnosis of unusual gliomas. It is very difficult to diagnose this rare condition based on imaging studies alone. However, when imaging findings are neither typical nor specific for a certain tumors, presumption of a rare condition is important and pathological confirmation is mandatory.
Figures and Tables
Fig. 1
Preoperative computed tomography shows a suspicious tumor mass on the right medial frontal lobe (A). Magnetic resonance imaging (MRI) revealed heterogeneous subtle enhancement on T1-weighted images (B and C). T2-weighted MRI showed well-circumscribed tumor mass with superficial siderosis (arrowheads) (D).
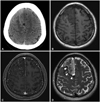
Fig. 2
The tumor is grayish and distinguished from normal brain by gross appearance (A). There was no continuation between tumor mass and surrounding normal brain. The tumor was totally removed (B), which was confirmed on immediate postoperative magnetic resonance imaging (C).
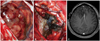
Fig. 3
Low power view shows relatively well-circumscribed tumor mass (A). High power view demonstrates glial tumor cells with increased cellularity (B). The majority of tumor cells are small round cells with a perinuclear halo, which is consistent with anaplastic oligodendroglioma (C). However, astrocytic tumor component was also observed in a small part of the tumor (D). A: H&E 12×. B: H&E 100×. C and D: H&E 400×.
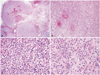
Fig. 4
By immunohistochemistry (IHC) stains, the tumor cells were strong positive for glial fibrillary acidic protein (GFAP) and Olig-2 (A, B). IHC also revealed that isocitrate dehydrogenase 1 (IDH1) was mutated (C) and epidermal growth factor receptor (EGFR) was amplified (D). Ki-67 proliferative index was about 10-15% (E). A: GFAP-IHC 200×. B: Olig-2-IHC 200×. C: IDH1-IHC 200×. D: EGFR-IHC 200×. E: Ki-67-IHC 200×.
References
1. Kakita A, Wakabayashi K, Takahashi H, Ohama E, Ikuta F, Tokiguchi S. Primary leptomeningeal glioma: ultrastructural and laminin immunohistochemical studies. Acta Neuropathol. 1992; 83:538–542.

2. Kalyan-Raman UP, Cancilla PA, Case MJ. Solitary, primary malignant astrocytoma of the spinal leptomeninges. J Neuropathol Exp Neurol. 1983; 42:517–521.

4. De Tommasi A, Occhiogrosso G, De Tommasi C, Luzzi S, Cimmino A, Ciappetta P. A polycystic variant of a primary intracranial leptomeningeal astrocytoma: case report and literature review. World J Surg Oncol. 2007; 5:72.

5. Cooper IS, Kernohan JW. Heterotopic glial nests in the subarachnoid space; histopathologic characteristics, mode of origin and relation to meningeal gliomas. J Neuropathol Exp Neurol. 1951; 10:16–29.

6. Keith T, Llewellyn R, Harvie M, Roncaroli F, Weatherall MW. A report of the natural history of leptomeningeal gliomatosis. J Clin Neurosci. 2011; 18:582–585.

7. Horoupian DS, Lax F, Suzuki K. Extracerebral leptomeningeal astrocytoma mimicking a meningioma. Arch Pathol Lab Med. 1979; 103:676–679.
8. Thomas JE, Falls E, Velasco ME, Zaher A. Diagnostic value of immunocytochemistry in leptomeningeal tumor dissemination. Arch Pathol Lab Med. 2000; 124:759–761.

9. Bailey P, Robitaille Y. Primary diffuse leptomeningeal gliomatosis. Can J Neurol Sci. 1985; 12:278–281.

10. Scully RE, Galdabini JJ, McNeely BU. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 17-1978. N Engl J Med. 1978; 298:1014–1021.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download