Abstract
Primary spinal cord melanoma is a rare central nervous system malignant tumor. Usually it resembles an intradural extramedullary (IDEM) nerve sheath tumor or melanoma. We experienced a patient with upper thoracic primary IDEM spinal cord melanoma who was diagnosed to be with hydrocephalus and without intracranial lesions. Initial symptoms of the patient were related to the hydrocephalus and the primary spinal cord melanoma was diagnosed eight months later. At the first operation, complete resection was impossible and the patient refused additional radiotherapy or chemotherapy. At 22 months after surgery, the patient revisited our institution with recurrent both leg weakness. Leptomeningeal dissemination was present in the whole spinal cord and only partial resection of tumor was performed. The symptoms slightly improved after surgery. Primary spinal cord melanoma is extremely rare but complete resection and additional radiotherapy or chemotherapy can prolong the disease free interval. Hydrocephalus or signs of increased intracranial pressure may be the diagnostic clue of spinal cord malignancy and progression.
Melanoma is a well known malignant tumor and early stage limited skin lesion is curable, but metastatic lesions are usually resistant to chemotherapy or conventional radiotherapy [1]. Melanoma develops mainly in the skin, and is rarely found in the central nervous system (CNS). An intradural spinal cord melanoma is rarer than a brain melanoma, and 27 surgical cases have been reviewed by Kim et al. [2]. Usually, spinal cord tumors including melanomas may cause myelopathy or radiculopathy by compression of the spinal cord or spinal nerve roots. However, spinal cord melanomas may disturb the cerebrospinal fluid (CSF) circulation, and increased intracranial pressure may occur. We experienced a patient with an intradural spinal cord melanoma in the upper thoracic spine with hydrocephalus and reported the case with a review of pertinent literature.
A 42-year-old man visited our outpatient clinic presenting with both leg weakness. Eight months before the visit, he had been diagnosed with elevated intraocular pressure accompanied by continuous headache and eyeball pain. Despite treatment for the glaucoma, the symptoms persisted and became aggravated. A week before admission, he experienced a seizure attack for several seconds, and a computed tomography scan at another hospital revealed hydrocephalus without intracranial causes. He complained of progressive both leg weakness, and sensory change below the T2 dermatome was found. He was then transferred to our institute. He had no other medical history. He had lost weight of more than 10 kg for the last 7 months. He complained of difficulties in urination and defecation. The neurologic examination showed hypoesthesia below the T2 and hyperactive deep tendon reflexes of the lower extremities.
Spinal magnetic resonance image (MRI) was done with contrast dye enhancement (Fig. 1). An intradural extramedullary (IDEM) mass lesion was found at the T2 level, ventral side to the spinal cord. The mass demonstrated a lower signal intensity than the spinal cord, and well enhancement of gadolinium in the T1-weighted image. The dorsal portion of the dura mater was slightly enhanced. There was no other lesion on the whole spine image. A brain MRI was performed, which showed no definite identifiable lesions.
Surgery was performed with respect to the benign IDEM tumor. A posterior midline approach was performed followed by a T2 laminoplasty. The dura matter was dark gray in color and was opened (Fig. 2), which showed a dark gray-colored mass that was severely adhered to the spinal cord and roots. After dissection, normal spinal cord was identified and the mass was removed as much as possible grossly. The black colored arachnoid membrane was also removed, but total resection was impossible. After decompression of the spinal cord, the dura was closed and sealed. There was no adverse event during the operation.
Histopathologic examination revealed a black or dark gray colored fragile mass in the gross view. Hematoyxlin and eosin stain of the tumor showed melanin pigmentation of tumor cells (Fig. 3). Immunohistochemical stain for human melanoma black revealed positive results for malignant melanoma. The MIB-1 index of 2.6%, atypia, and mitosis (0/10 in high power field) indicated that the melanoma was of intermediate grade.
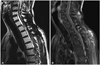
After surgery, positron emission tomography (PET) was performed for evaluation of the primary lesion but there were no demonstrable metastatic or other primary melanoma lesions beyond the spinal cord. There were no skin lesions on physical examination. During the hospitalization period after surgery, a seizure attack occurred which was a generalized tonic and clonic type. The cause for the seizure was suspected to be hydrocephalus, so a ventriculo-peritoneal (VP) shunt was planned. CSF study was done but no malignant cells were observed. However, the patient refused further treatment and was discharged. He also refused further adjuvant treatment. The patient revisited our hospital and was admitted again 22 months after the initial surgery with progressive both leg we-akness. During the period of follow-up loss, a VP shunt was performed in another hospital but the patient still had not received chemotherapy and radiotherapy for the melanoma. An MRI scan was done which showed that the spinal cord had swelled with dorsal side adhesion to the dorsal surface of the dura mater. The surface of the entire spinal cord was enhanced and this finding was regarded as leptomeningeal dissemination (Fig. 4). Revision surgery for decompression of the spinal cord was performed. There was more aggressive dissemination of the melanoma in the arachnoid and pia mater. There was severe adhesion between the melanin-pigmented tissue and neural tissues. Bleeding continued during removal of the melanoma and it was difficult to control. Total resection of the melanoma was impossible but decompression of the spinal cord was achieved. After the revision surgery, weakness of both legs had slightly improved but the patient refused additional treatment again and was discharged finally.
Primary CNS melanoma consists of approximately 1% of all melanomas and the accurate incidence of spinal cord melanomas has not been reported in the past literature [3]. Metastatic CNS melanoma is more frequent and has worse prognosis than primary CNS melanomas. Better prognosis of primary CNS melanoma has been reported after total excision and additional radiotherapy or chemotherapy [2]. In order to understand primary spinal cord melanomas more comprehensively, we discuss herein this tumor in terms of diagnosis, disease progression and treatment.
Primary spinal cord melanoma is often found as an IDEM tumor in preoperative imaging studies, mostly nerve sheath tumors or meningiomas [4]. Spinal cord melanomas do not have any distinguishable radiologic characteristics compared to other IDEM tumors [5]. Most surgeons identify spinal cord melanomas after opening of the dura mater by its unique black or dark gray color. After operation and pathologic diagnosis of the resected tumor, the patient may be examined for a primary melanoma site beyond the CNS by whole body surface examination or PET scan. Before surgery the primarily considered diagnosis may not be a melanoma because it is very hard to differentiate this tumor from other IDEM tumors. It originates from melanotic cells of the leptomeninges and mimics the general appearance of other nerve sheath tumors or meningiomas [6]. Preoperative diagnosis based on imaging studies is important for planning of surgical extent and additional treatment. Imaging data should be collected to detect diagnostic evidence of a primary spinal cord melanoma.
The clinical course of primary spinal cord melanomas is still unknown. Most melanomas have malignant features and finally causes direct dissemination or distant metastasis. This malignant tendency is not different from primary CNS melanomas or spinal cord melanomas, but reported cases showed various disease free periods from the first operation and subsequent radiotherapy or chemotherapy. In this patient, leptomeningeal dissemination had already been suspected at the time of the first operation because no other intracranial or spinal cause of headaches and ocular pressure elevation were found. A lesion of spinal cord was not usually suspected at the presentation of the first symptoms, therefore the diagnosis of a primary spinal cord melanoma may be delayed and leptomeningeal dissemination may progress before definitive diagnosis. Negative CSF study results did not support leptomeningeal dissemination, but sensitivity of the CSF study and MRI with gadolinium enhancement was not so different clinically, and radiologic diagnosis of leptomeningeal seeding may be acieved [7]. After the first operation, although the melanoma was partially removed, adjuvant radiotherapy or chemotherapy was not performed and leptomeningeal seeding may have progressed. Hydrocephalus may develop in an intracranial melanoma or other malignant tumors, but it occurs less frequently in a primary spinal cord melanoma. Of all reported cases of spinal cord melanomas, there has been no mention of the presence of hydrocephalus at initial diagnosis. However, congenital diseases such as neurocutaneous melanomatosis frequently accompanied with leptomeningeal seeding or dissemination has been reported [8].
The standard treatment for CNS melanoma is radiotherapy after surgical resection. Metastatic melanoma in the CNS resists adjuvant treatment and progresses rapidly, but primary spinal cord lesions often presents better prognosis than metastatic melanomas [9]. Larson et al. [3] reported 7 years as the life expectancy after surgery and radiotherapy. Moreover, other long-term survival periods of treated primary spinal cord melanoma cases have been shown in the literature [10,11]. The survival period is still unpredictable, but there have been some reports that total excision and postoperative radiotherapy may extend the survival period. Leptomeningeal seeding and hydrocephalus are poor prognostic factors in not only melanomas, but also in other spinal cord tumors, which means disease progression and difficulty in archiving total removal of the melanoma [12]. Hydrocephalus and letpomeningeal seeding were present in our patient at the time of initial diagnosis, so he had a high risk of disease progression and poor prognosis. Indeed, the patient refused radiotherapy and chemotherapy, and finally the melanoma metastasized to the whole spinal leptomeninges. Whether total resection of melanoma is achieved or not, radiation therapy should be provided for suppression of recurrence or progression of melanomas.
We report a patient with rare primary spinal cord melanoma which usually causes myelopathy or radiculopathy, but caused obstruction of CSF flow and consequential hydrocephalus in this case. This may lead to leptomeningeal seeding and poor prognosis, but lack of an adjuvant treatment after surgical resection may cause leptomeningeal progression. More cases of hydrocephalus in spinal cord tumors, especially in malignant tumors will be needed in further investigations.
Figures and Tables
Fig. 1
Preoperative spinal magnetic resonance image. T2-weighted image showed low signal intensity intradural extramedullary mass at T2-3 level (A). Well demarcated enhancing mass in T1-weighted image at T2-3 level was found. The enhancing thick membranous lesion was also found in the dorsal side of the spinal cord from T1 to T5 (B). In the axial view, the mass was located on the ventral side of the spinal cord in T1-weighted (C), T2-weighted (D), and T1-weighted image with gadolinium enhancement (E).
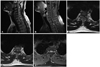
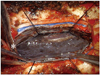
Fig. 2
Intraoperative gross photograph showed a black colored tumor which was diffuse leptomeningeal dissemination through the dorsal surface of the spinal cord. Asterisk (*) indicates cephalad direction.
References
1. Rubin KM. Management of primary cutaneous and metastatic melanoma. Semin Oncol Nurs. 2013; 29:195–205.

2. Kim MS, Yoon do H, Shin DA. Primary spinal cord melanoma. J Korean Neurosurg Soc. 2010; 48:157–161.

3. Larson TC 3rd, Houser OW, Onofrio BM, Piepgras DG. Primary spinal melanoma. J Neurosurg. 1987; 66:47–49.

4. Ryu DS, Park YM, Kim KH, Lee S, Kim KS. Primary intradural extramedullary malignant melanoma in the thoracic spine: case report and literature review. Korean J Spine. 2010; 7:184–187.
5. Farrokh D, Fransen P, Faverly D. MR findings of a primary intramedullary malignant melanoma: case report and literature review. AJNR Am J Neuroradiol. 2001; 22:1864–1866.
6. Lee CH, Moon KY, Chung CK, et al. Primary intradural extramedullary melanoma of the cervical spinal cord: case report. Spine (Phila Pa 1976). 2010; 35:E303–E307.
7. Straathof CS, de Bruin HG, Dippel DW, Vecht CJ. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol. 1999; 246:810–814.

8. Chu WC, Lee V, Chan YL, et al. Neurocutaneous melanomatosis with a rapidly deteriorating course. AJNR Am J Neuroradiol. 2003; 24:287–290.
9. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011; 117:1687–1696.

10. Nishihara M, Sasayama T, Kondoh T, Tanaka K, Kohmura E, Kudo H. Long-term survival after surgical resection of primary spinal malignant melanoma. Neurol Med Chir (Tokyo). 2009; 49:546–548.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download