Abstract
The best treatment for clival chordoma is obtained with total surgical excision, sometimes combined with adjuvant radiotherapy. A cerebrospinal fluid (CSF) fistula is a fatal complication that may occur following extended transsphenoidal surgery (TSS) and adjuvant radiotherapy. We report a case of fulminant meningitis without a CSF fistula in a 57-year-old woman who underwent TSS and multiple radiotherapies for a clival chordoma. She presented to our emergency room with copious epistaxis and odor inside her nasal cavity and had an unexpected fatal outcome. She was diagnosed with meningitis based on CSF culture and blood culture. While treating clival chordomas with adjuvant radiotherapy, clinicians should be aware of the possibility of fulminant meningitis.
Chordomas are rare, slow-growing malignant bony tumors, and it is one of the most difficult cranial tumors to treat, because it is not only difficult to remove totally due to its anatomical location, but also they have a high recurrence rate and can metastasize. They represent only 1-4% of intracranial tumors [1]. Current management of clival chordomas is surgical-wide en bloc resection, radiotherapy, or both [2].
Anterior skull base approaches may be useful for clival tumors. Among these, transsphenoidal surgery (TSS) is preferred for tumors located along the mid and upper portions of the clivus. But, TSS has several disadvantages, such as limited tumor exposure, a deep and narrow surgical view, the occurrence of a postoperative cerebrospinal fluid (CSF) fistula, and meningitis [1]. Also, CSF fistulas and meningitis are the most common fatal complications [3]. Thus, surgical treatment has been the most useful strategy, but total surgical resection of a chordoma is challenging.
Recurrence is common without total surgical resection, and adjuvant radiotherapy is required for unresectable and recurred lesions [1]. Furthermore, complications of radiotherapy have been reported in previous reports, such as cranial neuropathy, hypopituitarism, brain necrosis, and hemorrhage from the internal carotid artery. In contrast, fulminant meningitis has rarely been reported in patients after radiotherapy [4,5]. We report a rare case of fulminant meningitis without a CSF fistula after TSS and radiotherapy for a clival chordoma.
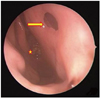
A 57-year-old woman presented with visual disturbance, diplopia and headaches. She had visited another hospital in October 2009. Magnetic resonance imaging (MRI) showed an osteolytic, irregular enhancing lesion occupying the clivus on T1-weighted gadolinium-enhanced images (Fig. 1A). She had undergone TSS in October 2009 (Fig. 1B). Microscopically, the excisional biopsy was consistent with a chordoma with chondroid differentiation, which was not totally removed. After one year, she presented with diplopia again, and an MRI showed that the clival chordoma had increased in size (Fig. 2A). Extended TSS was performed again in December 2010 (Fig. 2B), but the tumor still remained. At that time, postoperative CSF leakage was not observed because the dura was not opened during the operation. The tumor had increased again on follow-up MRI in May 2011. She underwent Gamma knife radiosurgery (Elekta, Stockholm, Sweden) in May 2011. The treatment dose was 18.5 Gy at the tumor margin (50% isodose). In December 2012, she underwent proton beam radiotherapy (IBA, Brussels, Belgium) for the increased tumor size on the follow-up MRI. The treatment dose was 6,960 cGy in 29 fractions. After 3 months, she occasionally presented with an unpleasant odor inside her nasal cavity. She visited the radiation-oncology department several times and only took antibiotic drugs and steroids, but unfortunately the symptoms did not resolve. All of her treatments were performed in another hospital at that time. She visited our emergency medical team for the first time with copious epistaxis and an odor inside her nasal cavity in June 2013. She had an systemic inflammatory response syndrome on admission: her body temperature was 38.2 degrees and the white blood cell (WBC) was 30,040/mm3. First, we performed an imaging study to identify the cause of the epistaxis, because she had a history of TSS in another neurosurgery institution. While performing a brain computed tomography (CT) scan, the patient showed comatose mental state and presented with low blood pressure due to septic shock. After cardiopulmonary resuscitation was performed, her vital signs recovered. However, the subsequent CT did not reveal the cause of the comatose mental state. Therefore, we performed further evaluations in the intensive care unit to ascertain light on her clinical course. We examined the CSF examination by lumbar puncture and performed MRI without enhanced gadolium because her vital signs were unstable at that time (Fig. 3). An exploration inside the nasal cavity with a nasal endoscope showed synthetic materials used in the reconstruction of the sellar floor during a previous operation, a nasal septum defect due to necrosis. We also observed a pale and fibrotic mucous membrane due to the radiotherapy. The scarring and narrowing of the blood vessels within the area that received the treatment had caused disruption and necrosis of this membrane. CSF rhinorrhea was not observed (Fig. 4). The tissue was not biopsied but Enterococcus avium and Escherichia coli were identified on the mucosal culture. The CSF examination revealed an increased WBC count (11,280 cells/mm3) and protein level (345 mg/dL) with a 4 mg/dL glucose level. Enterococcus avium and Escherichia coli were identified on the CSF culture and blood culture. We diagnosed the patient to be with bacterial meningitis and immediately administered antibiotic therapy. Despite the antibiotic therapy, the patient expired a week later.
TSS is a useful and safe approach, but complications of TSS such as central nervous system injury, or meningitis related to CSF fistulas and other complications have been reported in the literature in numerous studies [6]. CSF fistulas and meningitis are serious complications associated with TSS [7]. However, our patient did not present with a postoperative CSF fistula because the tumor was located in the extradural space and surgery was performed without injuring the dura.
Radiotherapy is relatively free of immediate complications, but adverse effects of radiation depend on the radiosensitivity of the body sites being treated, the volume of normal tissue irradiated, the total doses, and the rate of dose accumulation. Side effects are most evident in rapidly proliferating tissues, such as the skin and mucosa. Irradiated soft tissue becomes thin, hypovasculized and easily injured by any slight trauma or infection. Also, the tissue heals slowly, and tissue damage may persist for several years [8]. In the treatment for chordomas, recurrence free survival was significantly improved with greater extent of resection and with proton beam radiotherapy, but conventional radiotherapy did not appear to have an effect on survival [2]. This patient had undergone multiple radiotherapies for a recurrent clival chordoma. We think retrospectively that the total therapeutic dose of her radiotherapy was more than the proper and safe therapeutic dose of 70 to 80 Gy that can now be given safely without a high risk of injury to surrounding structures. The arachnoid membrane, dura mater and regenerative mucous membrane of the sphenoidal sinus can easily be disrupted by over-doses of radiation damage, which can lead to direct infection and cause meningitis, which may be rapid in onset and can be fulminant. The complications of radiotherapy may be attributed to many factors, such as previous surgery and radiotherapy and advanced age, or other conditions of patient [9]. Therefore, appropriate radiation therapeutic doses to prevent complications may be difficult to define.
Bacterial meningitis is relatively uncommon after neurosurgical procedures but the mortality rate may exceed 20% if treatment is delayed. To prevent the surgical site defects which can induce CSF fistulas associated with meningitis before radiotherapy during TSS, preservation of the sphenoid mucous membrane and minimization of the mucosal resection is essential. Before radiotherapy, the condition of the surgical lesion should be checked to determine whether it has healed sufficiently. If it has not healed enough, then radiotherapy should be delayed because chordomas are generally considered to be slow growing tumors [1,2,10]. If the mucous membrane has not healed, the reconstruction of the sellar floor and sphenoid sinus mucous should be rescheduled for a later date. During the mucosal reconstruction surgery, non-irradiated tissue should be used, because irradiated tissue is considered as problematic in terms of viability. Non-irradiated tissue, such as the subumbilical fascia, muscle, and fat away from the irradiated sphenoid sinus mucous, should be used instead as reconstruction tissue. Conservative treatments, such as a longterm wound dressing, and antibiotics usage are also needed.
Several factors, such as prolonged steroid therapy, irradiation or chemotherapy, repeat operations, infection, and malnutrition can predispose patients to poor wound healing and lead to meningitis. Our patient had several risk factors for meningitis. First, she presented with sinusitis in December 2010 before undergoing a second extended TSS. In addition, 3 months after the proton beam radiotherapy, she presented with an unpleasant odor inside her nasal cavity and was treated with prolonged steroid therapy. She may have had chronic sinusitis. In our opinion, sinusitis and radiotherapy may have irritated the previous surgical mucous site, and the destruction of the previous lesion after radiotherapy may have led to the copious epistaxis. Second, she underwent sellar floor reconstruction with synthetic materials during extended TSS. These materials also increase the incidence of fulminant sinusitis and delay wound healing. Prevention of these complications by reconstruction with vascularized graft can be helpful [8]. Regarding the aforementioned risk factors, her fatal course was caused by fulminant meningitis due to vulnerability to inflammation following radiotherapy.
In the literature, the postoperative meningitis interval to the appearance of the first symptoms such as fever, headache, meningism varied from 1 to 42 days after TSS. In this case, as mentioned above, the patient underwent extended TSS without complications, and adjuvant radiotherapy presented delayed bacterial meningitis several months after the surgery. Our patient was prescribed antibiotics and steroids in the radiation-oncology outpatient department but she still presented with sinusitis that had not improved. Her sinusitis subsequently worsened and infected the adjacent mucosal membranes, and this course led to the fatal meningitis. Therefore, patients who have risk factors for meningitis require long-term follow-up to monitor the presence of CSF fistulas and meningitis, and close cooperation is also essential with other departments to prevent the delayed diagnosis of meningitis after radiotherapy.
In conclusion, while treating tumors with adjuvant radiotherapy after skull base surgery, we should be aware of the possibility of fulminant meningitis due to delayed radiation damage to the mucous membranes. Radiotherapy decreases tissue viability and increases the possibility of tissue necrosis and infection. Before adjuvant radiotherapy, we should pay special consideration to the use of proper graft material and repair techniques necessary during skull base surgery. Radiotherapy should balance the expected outcome with the side effects.
Figures and Tables
Fig. 1
Preoperative and postoperative MR images of the first transsphenoidal surgery. A: Preoperative contrast-enhanced sagittal T1-weighted image showing a lesion of the clivus, with destruction of the bony margins of the clivus. The lesion is found just anterior to the brainstem. B: Postoperative contrast-enhanced sagittal T1-weighted image after the tumor was removed.
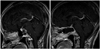
Fig. 2
Following MR image when the patient presented with diplopia again 14 months after the first surgery and its postoperative image. A: Contrast-enhanced sagittal T1-weighted image showing evidence of recurrent tumor. B: Contrast-enhanced sagittal T1-weighted image after the second extended transsphenoidal surgery with still remained tumors in clivus and multifocal peripheral lesions.

References
1. Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012; 13:e69–e76.

2. St Martin M, Levine SC. Chordomas of the skull base: manifestations and management. Curr Opin Otolaryngol Head Neck Surg. 2003; 11:324–327.

3. Kono Y, Prevedello DM, Snyderman CH, et al. One thousand endoscopic skull base surgical procedures demystifying the infection potential: incidence and description of postoperative meningitis and brain abscesses. Infect Control Hosp Epidemiol. 2011; 32:77–83.

4. Hongmei Y, Zhe W, Jing W, Daokui W, Peicheng C, Yongjie L. Delayed cerebrospinal fluid rhinorrhea after gamma knife surgery in a patient with a growth hormone-secreting adenoma. J Clin Neurosci. 2012; 19:900–902.

5. Kim CH, Chung SK, Dhong HJ, Lee JI. Cerebrospinal fluid leakage after gamma knife radiosurgery for skull base metastasis from renal cell carcinoma: a case report. Laryngoscope. 2008; 118:1925–1927.

6. Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997; 40:225–236. discussion 236-7.

7. Siedek V, Pilzweger E, Betz C, Berghaus A, Leunig A. Complications in endonasal sinus surgery: a 5-year retrospective study of 2,596 patients. Eur Arch Otorhinolaryngol. 2013; 270:141–148.

8. Dormand EL, Banwell PE, Goodacre TE. Radiotherapy and wound healing. Int Wound J. 2005; 2:112–127.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download