Abstract
Alzheimer's disease (AD) is characterized by the accumulation of amyloid beta (Aβ) and the hyperphosphorylation of tau protein in the brain, leading to the increase in inflammation and neuronal loss. Recently, evidences to support the association between type 2 diabetes mellitus (T2DM) and AD have markedly increased by clinical researches and experimental studies. Reduced insulin action and impaired glucose metabolism in the brain leads to diabetes induced AD. Androgen, a male sex hormone, was known to regulate inflammatory response, Aβ deposition in AD, insulin signaling, and synaptic plasticity in brain. Clinical studies demonstrated that androgen deficiency results in the increased risk of AD and its severe progression in male subjects. We reviewed the significant evidences to support that low testosterone levels are linked to diabetes-induced AD based on previous studies. Thus, we highlight the therapeutic potential of androgen in diabetes induced AD.
Alzheimer's disease (AD) is characterized by the accumulation of amyloid beta (Aβ) protein, hyperphosphorylated tau protein, and synaptic and neuron loss in the brain.12 Recently, AD is most common neurodegenerative disorder that influences 5 million people in the United States.34 Current studies have reported that type 2 diabetes mellitus (T2DM) is directly associated with the increased risk of AD56 and cognitive dysfunction.7 Elevated glycemia in normal subjects leads to the increased risk of AD, and also triggers cognitive decline and decreased hippocampal volume.89 Moreover, AD incidence is positively correlated with insulin resistance which leads to T2DM.10 Furthermore, sex differences affect the onset of AD, with women accounting for two-thirds of patients with AD.11 In addition, women shows greater severity for AD and mild cognitive impairment compared to men.121314 Generally, AD patients show decreased levels of testosterone in the brain.15 Clinically, the use of androgen depletion therapy leads to increased plasma levels of Aβ in the brain,161718 and lower serum testosterone levels were found in the AD patients compared to non-AD controls.1920 Androgen as a sex hormone has been known to activate a variety of reproductive behavior, and regulate emotion.21 mRNA expressions of androgen receptor were found in the central nervous system (CNS) including forebrain, midbrain, and spinal cord.222324 In CNS, androgen have multiple functions such as neuroprotective function, neurite growth, neurogenesis, and neuronal survival,25 and regulate physiological functions including emotion and cognition.262728 Based on the previous researches, this review summarizes recent evidences regarding the role of androgen in diabetic induced AD, suggesting that androgen is associated with glucose metabolism, insulin signaling and cognitive decline in the brain.
In the T2DM, the impairment of glycemic control and insulin resistance triggers mild cognitive impairment (MCI).2930 Recent study with functional magnetic resonance imaging reported that patients with T2DM have changed neuronal activity in broad brain regions related with cognitive function.31 Imaging studies showed increase of brain atrophy in patients with T2DM which is associated with cognitive decline.3233 Also, the T2DM rat model showed the pathology of AD including neuronal loss, increased Aβ level, and hyperphosphorylated tau.3435 In the insulin resistance induced AD transgenic mice, high fat diet triggers increase of Aβ accumulation and decrease of insulin degradation enzyme (IDE).36 APP/PS1 transgenic mice with administration of sucrose-supplemented water showed glucose intolerance, elevation of insulin levels, increase of Aβ deposition, and memory dysfunction.37 Dysregulation of insulin and glucose have been linked to increases of AD pathology,38 and the expression of insulin receptor was observed in the AD brain.3940 Several clinical studies show that the treatment of T2DM leads to the improvement of AD pathologies such as cognitive deficit41 by decreasing Aβ levels4243 and tau pathologies.44 One study demonstrated that diet induced obesity in the triple-transgenic mice model of AD showed excessive Aβ burden, and aggravation of behavior dysregulation.45 Another study reported that the metabolic dysfunction and the acceleration of AD related pathologies were found in the insulin receptor substrate 2 knockout AD mice model.4647 Based on the previous evidences, insulin resistance, glucose deregulation, and inflammation may be in the central mechanisms between the onset and progression of T2DM and AD.
Androgen is a sex steroid hormone in men which exerts multiple physiological functions leading to maturation of reproductive systems.48 Testosterone, dihydrotestosterone (DHT), androstenedione, dehydroepiandrosterone (DHEA) and its sulphate (DHEAS) have been known as major androgens in males, and play an critical role in cognition, and their RNAs were observed in the AD cerebral cortex and hippocampus.22 Several studies reported that androgens play an important role in memory function in men, and low androgen level is directly linked to the onset of dementia,4950515253 thus androgen deficiency could lead to neural dysfunction in mood and cognition.5455 Low levels of free testosterone were also observed in the brain of AD patients.56 Furthermore, low levels of testosterone in blood57 or brain1551 lead to the increased risk for developing AD.15 A strong association between low testosterone levels and insulin resistance was observed in men.5859 In addition, low levels of testosterone were commonly found in the T2DM patients compared with non-diabetic controls.58 Therefore, therapy using testosterone may result in the reduction of T2DM features.6061 Also, androgen deprivation therapy has shown reduced insulin sensitivity and glycemic control.62 Considering the previous evidences, low levels of testosterone may contribute to insulin resistance, and glucose deregulation in the AD brain.
The accumulation of amyloid plaques is the neuropathological hallmarks of AD.63 According to the study which investigated the regulatory function of androgens on Aβ,64 APP metabolism and Aβ production in cortical neurons were regulated by testosterone levels in the brain.516566 As mention above, androgen could modulate Aβ production in the AD brain. Furthermore, androgen contributes to the increase of neuronal viability in brain.676869 Androgen depleted rats exhibited severe neuronal loss in the pyramidal layer of hippocampus70 as well as under oxidative stress71 or Aβ toxicity condition.7273 One study demonstrated that androgens could activate PI3K signaling through intracellular androgen receptor,7475 and ERK/CREB signaling in neurons.7677 Testosterone could modulate neuronal damage under oxidative stress condition,71 and reduce neuronal apoptosis and autophagic cell death.78 Testosterone reduces neuronal apoptosis through the phosphorylation of ERK as well as through the activation of Bcl2 which increases neuron viability.79 Collectively, androgen could protect neuronal cell damage against oxidative stress in brain.
Cross-sectional studies have reported an inverse association between testosterone levels and insulin resistance in men.80 Increased insulin resistance was triggered in men with a 25% decrease of serum testosterone compared to normal men.81 Acute androgen deprivation could attenuate insulin sensitivity in healthy men,82 and reduction of total and free serum testosterone could lead to T2DM in men.83 Moreover, synaptic plasticity is a critical issue in learning and memory function.84 In the cerebral cortex of AD patients, alteration of synaptic plasticity occurs prior to formation of Aβ, and ultimately triggers cognitive deficits.84 It has been reported that androgen receptors are involved in memory and learning by involving synapse function.8586 DHT could reduce the binding of N-methyl-D-aspartate (NMDA) receptor antagonist in hippocampus CA1 region,87 and control NMDA mediated depolarization in pyramidal neurons.88 Recent study reported that androgen inhibits reduction of hippocampal dendritic spine density in rats.89 Another study reported that testosterone promotes hippocampal synaptogenesis.90 Also, testosterone could enhance the expression of brain derived neurotrophic factor, leading to improvement in hippocampal dendritic spine density, and the phosphorylation of CREB to enhance synaptic plasticity.86 Androgens improve spine-synapse density in the CA1 of rodents.91 Taken together, androgen could improve insulin resistance by binding androgen receptor in brain, enhance synaptic failure, and promote synaptogenesis in the AD brain.
Here, we reviewed the role of androgen in diabetes induced AD. We highlight the therapeutic potential of androgen in diabetes induced AD brain including the improvement of insulin resistance, the inhibition of Aβ accumulation, the protection of neuronal cells against oxidative stress, and the suppression of synaptic dysfunction (Fig. 1). Given that testosterone supplementation could improve cognitive decline92 and memory function,939495 we speculate that androgen can be a good target hormone to alleviate the neuropathology in diabetes induced AD.
Figures and Tables
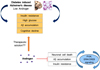 | Fig. 1Systemic images about the relationship between diabetes induced Alzheimer's disease (AD) and androgen. Diabetes induced is charactered by insulin resistance, high glucose level, Aβ accumulation, and cognitive decline. Androgen could protect neuronal cell death, Aβ accumulation, and insulin resistance in brain through PI3K, and ERK/CREB signaling. This image shows the therapeutic possibility of androgen in diabetes induced AD. |
ACKNOWLEDGEMENTS
This study was supported by the Brain Research Program through the National Research Foundation of Korea funded by a grant from 2016R1D1A1B03930394.
References
1. Mucke L, Selkoe DJ. Neurotoxicity of amyloid betaprotein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012; 2:a006338.
2. Zempel H, Mandelkow E. Lost after translation: missorting of Tau protein and consequences for Alzheimer disease. Trends Neurosci. 2014; 37:721–732.

3. Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014; 11:98.

4. Morris GP, Clark IA, Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol Commun. 2014; 2:135.

5. Huang CC, Chung CM, Leu HB, Lin LY, Chiu CC, Hsu CY, et al. Diabetes mellitus and the risk of Alzheimer's disease: a nationwide population-based study. PLoS One. 2014; 9:e87095.

6. Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, Iwaki T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011; 77:1126–1134.

7. Cha DS, Carvalho AF, Rosenblat JD, Ali MM, McIntyre RS. Major depressive disorder and type II diabetes mellitus: mechanisms underlying risk for Alzheimer's disease. CNS Neurol Disord Drug Targets. 2014; 13:1740–1749.

8. Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013; 369:1863–1864.

9. Kerti L, Witte AV, Winkler A, Grittner U, Rujescu D, Flöel A. Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology. 2013; 81:1746–1752.

10. Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, et al. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010; 75:764–770.

11. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013; 80:1778–1783.

12. Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y). 2015; 1:103–110.

13. Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005; 62:685–691.

14. Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer's disease: a meta analysis. J Clin Exp Neuropsychol. 2012; 34:989–998.

15. Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging. 2011; 32:604–613.

16. Gandy S, Almeida OP, Fonte J, Lim D, Waterrus A, Spry N, et al. Chemical andropause and amyloid-beta peptide. JAMA. 2001; 285:2195–2196.
17. Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, et al. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem. 2003; 87:1052–1055.
18. George S, Petit GH, Gouras GK, Brundin P, Olsson R. Nonsteroidal selective androgen receptor modulators and selective estrogen receptor beta agonists moderate cognitive deficits and amyloid-beta levels in a mouse model of Alzheimer's disease. ACS Chem Neurosci. 2013; 4:1537–1548.

19. Watanabe T, Koba S, Kawamura M, Itokawa M, Idei T, Nakagawa Y, et al. Small dense low-density lipoprotein and carotid atherosclerosis in relation to vascular dementia. Metabolism. 2004; 53:476–482.

20. Chu LW, Tam S, Lee PW, Wong RL, Yik PY, Tsui W, et al. Bioavailable testosterone is associated with a reduced risk of amnestic mild cognitive impairment in older men. Clin Endocrinol (Oxf). 2008; 68:589–598.

22. Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990; 294:76–95.

23. Yu WH, McGinnis MY. Androgen receptors in cranial nerve motor nuclei of male and female rats. J Neurobiol. 2001; 46:1–10.

24. Bezdickova M, Molikova R, Bebarova L, Kolar Z. Distribution of nuclear receptors for steroid hormones in the human brain: a preliminary study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007; 151:69–71.

25. Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009; 89:134–152.

26. Melcangi RC, Mensah-Nyagan AG. Neurosteroids: measurement and pathophysiologic relevance. Neurochem Int. 2008; 52:503–505.

27. Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001; 37:201–222.

28. Darnaudéry M, Pallarès M, Piazza PV, Le Moal M, Mayo W. The neurosteroid pregnenolone sulfate infused into the medial septum nucleus increases hippocampal acetylcholine and spatial memory in rats. Brain Res. 2002; 951:237–242.

29. Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging. 2006; 10:293–295.
30. Stolk RP, Breteler MM, Ott A, Pols HA, Lamberts SW, Grobbee DE, et al. Insulin and cognitive function in an elderly population. The Rotterdam Study. Diabetes Care. 1997; 20:792–795.

31. Xia W, Wang S, Sun Z, Bai F, Zhou Y, Yang Y, et al. Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology. 2013; 38:2493–2501.

32. Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013; 36:4036–4042.

33. Brands AM, Kessels RP, Hoogma RP, Henselmans JM, van der, Kappelle LJ, et al. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes. 2006; 55:1800–1806.

34. Jung HJ, Kim YJ, Eggert S, Chung KC, Choi KS, Park SA. Age-dependent increases in tau phosphorylation in the brains of type 2 diabetic rats correlate with a reduced expression of p62. Exp Neurol. 2013; 248:441–450.

35. Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, et al. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007; 27:13635–13648.

36. Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004; 18:902–904.

37. Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007; 282:36275–36282.

38. Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000; 24:855–872.

39. Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005; 7:63–80.

40. Zhao L, Teter B, Morihara T, Lim GP, Ambegaokar SS, Ubeda OJ, et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer's disease intervention. J Neurosci. 2004; 24:11120–11126.

41. Luchsinger JA, Palmas W, Teresi JA, Silver S, Kong J, Eimicke JP, et al. Improved diabetes control in the elderly delays global cognitive decline. J Nutr Health Aging. 2011; 15:445–449.

42. Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via upregulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009; 106:3907–3912.

43. Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012; 69:29–38.

44. Searcy JL, Phelps JT, Pancani T, Kadish I, Popovic J, Anderson KL, et al. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2012; 30:943–961.

45. Barron AM, Rosario ER, Elteriefi R, Pike CJ. Sex-specific effects of high fat diet on indices of metabolic syndrome in 3xTg-AD mice: implications for Alzheimer's disease. PLoS One. 2013; 8:e78554.

46. Freude S, Hettich MM, Schumann C, Stöhr O, Koch L, Köhler C, et al. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer's disease. FASEB J. 2009; 23:3315–3324.

47. Killick R, Scales G, Leroy K, Causevic M, Hooper C, Irvine EE, et al. Deletion of Irs2 reduces amyloid deposition and rescues behavioural deficits in APP transgenic mice. Biochem Biophys Res Commun. 2009; 386:257–262.

48. Yu IC, Lin HY, Sparks JD, Yeh S, Chang C. Androgen receptor roles in insulin resistance and obesity in males: the linkage of androgen-deprivation therapy to metabolic syndrome. Diabetes. 2014; 63:3180–3188.

49. English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000; 21:890–894.

50. Moffat SD. Effects of testosterone on cognitive and brain aging in elderly men. Ann N Y Acad Sci. 2005; 1055:80–92.

51. Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004; 292:1431–1432.

52. Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer's disease. Neuroendocrinol Lett. 2001; 22:163–168.
53. Rosario ER, Pike CJ. Androgen regulation of beta-amyloid protein and the risk of Alzheimer's disease. Brain Res Brain Res Rev. 2008; 57:444–453.

56. Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer's disease. Exp Gerontol. 2004; 39:1633–1639.

57. Rasmuson S, Näsman B, Carlström K, Olsson T. Increased levels of adrenocortical and gonadal hormones in mild to moderate Alzheimer's disease. Dement Geriatr Cogn Disord. 2002; 13:74–79.

58. Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008; 93:1834–1840.

59. Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007; 30:911–917.

60. Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007; 156:595–602.

61. Bhasin S, Parker RA, Sattler F, Haubrich R, Alston B, Umbleja T, et al. Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab. 2007; 92:1049–1057.

62. Haidar A, Yassin A, Saad F, Shabsigh R. Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male. 2007; 10:189–196.

63. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002; 297:353–356.

64. Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, et al. Testosterone reduces neuronal secretion of Alzheimer's beta-amyloid peptides. Proc Natl Acad Sci U S A. 2000; 97:1202–1205.
65. Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer's disease. J Neurosci. 2006; 26:13384–13389.

66. Iwata N, Higuchi M, Saido TC. Metabolism of amyloid-beta peptide and Alzheimer's disease. Pharmacol Ther. 2005; 108:129–148.
67. Lund TD, Salyer DL, Fleming DE, Lephart ED. Pre- or postnatal testosterone and flutamide effects on sexually dimorphic nuclei of the rat hypothalamus. Brain Res Dev Brain Res. 2000; 120:261–266.

68. Yang SL, Chen YY, Hsieh YL, Jin SH, Hsu HK, Hsu C. Perinatal androgenization prevents age-related neuron loss in the sexually dimorphic nucleus of the preoptic area in female rats. Dev Neurosci. 2004; 26:54–60.

69. Tetzlaff JE, Huppenbauer CB, Tanzer L, Alexander TD, Jones KJ. Motoneuron injury and repair: new perspectives on gonadal steroids as neurotherapeutics. J Mol Neurosci. 2006; 28:53–64.

70. Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003; 122:573–578.

71. Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001; 892:255–262.

72. Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005; 94:1639–1651.

73. Park SY, Tournell C, Sinjoanu RC, Ferreira A. Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons. Neuroscience. 2007; 144:119–127.

74. Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006; 147:2028–2034.

75. Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007; 148:2458–2464.

76. Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci U S A. 2004; 101:10919–10924.

77. Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, et al. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004; 64:7156–7168.

78. Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001; 77:1319–1326.

79. Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009; 30:239–258.

80. Simon D, Preziosi P, Barrett-Connor E, Roger M, Saint-Paul M, Nahoul K, et al. Interrelation between plasma testosterone and plasma insulin in healthy adult men: the Telecom Study. Diabetologia. 1992; 35:173–177.

81. Kupelian V, Hayes FJ, Link CL, Rosen R, McKinlay JB. Inverse association of testosterone and the metabolic syndrome in men is consistent across race and ethnic groups. J Clin Endocrinol Metab. 2008; 93:3403–3410.

82. Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007; 92:4254–4259.

83. Stanworth RD, Jones TH. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front Horm Res. 2009; 37:74–90.

84. Liu L, Orozco IJ, Planel E, Wen Y, Bretteville A, Krishnamurthy P, et al. A transgenic rat that develops Alzheimer's disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiol Dis. 2008; 31:46–57.

85. Jia J, Kang L, Li S, Geng D, Fan P, Wang L, et al. Amelioratory effects of testosterone treatment on cognitive performance deficits induced by soluble Abeta1-42 oligomers injected into the hippocampus. Horm Behav. 2013; 64:477–486.

86. Jia JX, Cui CL, Yan XS, Zhang BF, Song W, Huo DS, et al. Effects of testosterone on synaptic plasticity mediated by androgen receptors in male SAMP8 mice. J Toxicol Environ Health A. 2016; 79:849–855.

87. Kus L, Handa RJ, Hautman JM, Beitz AJ. Castration increases [125I]MK801 binding in the hippocampus of male rats. Brain Res. 1995; 683:270–274.

88. Pouliot WA, Handa RJ, Beck SG. Androgen modulates N-methyl-D-aspartate-mediated depolarization in CA1 hippocampal pyramidal cells. Synapse. 1996; 23:10–19.

89. Li S, Kang L, Zhang C, Xie G, Li N, Zhang Y, et al. Effects of dihydrotestosterone on synaptic plasticity of hippocampus in male SAMP8 mice. Exp Gerontol. 2013; 48:778–785.

90. Frye CA, Edinger KL, Seliga AM, Wawrzycki JM. 5alpha-reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendocrinology. 2004; 29:1019–1027.

91. Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004; 24:495–499.

92. Giagulli VA, Guastamacchia E, Licchelli B, Triggiani V. Serum testosterone and cognitive function in ageing male: updating the evidence. Recent Pat Endocr Metab Immune Drug Discov. 2016; 10:22–30.

93. Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004; 29:1071–1081.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download