Abstract
Bile acid has been well known to serve as a hormone in regulating transcriptional activity of Farnesoid X receptor (FXR), an endogenous bile acid nuclear receptor. Moreover, bile acid regulates diverse biological processes, including cholesterol/bile acid metabolism, glucose/lipid metabolism and energy expenditure. Alteration of bile acid metabolism has been revealed in type II diabetic (T2D) patients. FXR-mediated bile acid signaling has been reported to play key roles in improving metabolic parameters in vertical sleeve gastrectomy surgery, implying that FXR is an essential modulator in the metabolic homeostasis. Using a genetic mouse model, intestinal specific FXR-null mice have been reported to be resistant to diet-induced obesity and insulin resistance. Moreover, intestinal specific FXR agonism using gut-specific FXR synthetic agonist has been shown to enhance thermogenesis in brown adipose tissue and browning in white adipose tissue to increase energy expenditure, leading to reduced body weight gain and improved insulin resistance. Altogether, FXR is a potent therapeutic target for the treatment of metabolic diseases.
Bile acids are converted from cholesterol in the liver by numerous cytochrome P450 enzymes, including cholesterol-7α-hydroxylase (CYP7A1), steroid 7α-hydroxylase (CYP27A1) and sterol-27-hydroxylase (CYP27A1).1 Bile acids have been well known to serve as digestive juice to emulsify lipid and promote lipid absorption in the intestinal tract. After endogenous bile acid nuclear receptor Farnesoid X Receptor (FXR) has been reported,23 bile acids are now widely accepted as metabolic regulator to control whole body homeostasis, such as glucose/lipid metabolism and energy expenditure. Thus, the discovery of endogenous bile acid nuclear receptor FXR proposes new perspectives to understand molecular mechanisms and physiological roles of bile acids and their receptors in various tissues to maintain whole body homeostasis.
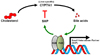
FXR belongs to nuclear receptor superfamily that contains both DNA and ligand binding domains.456 With absence of ligand, nuclear receptors generally bind to their responsive elements with corepressors, including Silencing Mediators of Retinoic acid and Thyroid hormone receptor (SMRT) and histone deacetylases (HDACs) and suppresses their target gene expressions. Upon binding with their ligands, nuclear receptors interact with numerous coactivators and induce their target gene expressions (Fig. 1). FXR heterodimerizes with retinoid X receptor (RXR) and binds to the FXR response element which is localized in the promoter region of FXR target genes.4567 FXR is expressed in various tissues, including liver, adrenal glands, intestine, adipose tissue, endothelial wall, pancreas and kidney.6
Bile acids play as endogenous FXR ligands with high affinity. Both free and conjugated bile acids are able to activate FXR. A well-known hydrophobic bile acid, chenodeoxycholic acid (CDCA) is the most efficacious ligand of FXR (EC50=10µM).289 Generally, the order of potency of bile acids is CDCA>lithocholic acid (LCA)=deoxycholic acid (DCA)>cholic acid (CA).9 Interestingly, hydrophilic bile acids, including ursodeoxycholic acid (UDCA) and muricholic acid (MCA) were not able to activate FXR.10 A synthetic bile acid derivative, 6α-ethylchenodeoxycholic acid (6-ECDCA) has been developed and reported with a higher affinity with FXR than the natural bile acids.9 6-ECDCA recently has been renamed as obeticholic acid, which has recently been approved as a therapeutic reagent for primary biliary cirrhosis (PBC) by Food and Drug Administration,1112 suggesting that FXR is a therapeutic potential target for other metabolic diseases.
Bile acids are generally conjugated to taurine or glycine upon synthesis. After conjugation, bile acids are secreted into the bile canaliculi. Upon food intake, feeding signal stimulates bile acid secretion into small intestine. Mostly, 95% of secreted bile acids are recycled by entero-hepatic circulation; The remaining 5% of the bile acids excluded in each entero-hepatic circulation. Therefore, the conversion of bile acid from cholesterol compensates an equivalent amount of bile acids to maintain a constant bile acid pool size in the system.
The recycle of 95% of bile acids via entero-hepatic circulation requires the apical sodium-dependent bile salt transporter (ASBT).681314 After transporting bile acids inside enterocytes by ASBT, bile acids are bound by the intestinal bile acid-binding protein (IBABP) which is critical for entero-hepatic circulation of bile acids. Besides ASBT, organic solute transporter alpha and beta (OSTα and β) transport bile acids to the blood vessel, the portal vein.14 Thus, elevated level of bile acids in the intestine induces expression of genes in bile acid transporters to transport bile acids from the intestine to the liver.
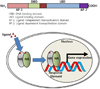
As properties of bile acids are detergent to emulsify lipid substance, accumulation of bile acids in the hepatocytes leads to hepatotoxicity. Bile acid synthesis occurs in the hepatocytes and comprises two pathways: there are two key enzymes, such as CYP7A1 and CYP27A1, respectively.15 Upon FXR activation by bile acids, FXR induces gene expression of small heterodimer partner (SHP), which suppresses gene expression of CYP7A1 to reduce the rate of hepatic bile acid synthesis via negative feedback (Fig. 2).571516
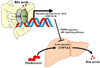
Besides CYP7A1 suppression by SHP, bile acid signaling from intestinal is able to regulate hepatic bile acid synthesis.17 Secretion of bile acids into small intestine activates transcriptional activity of FXR in the intestine, leading to induction of fibroblast growth factor 15/19 (FGF15/19) gene expression as well as bile acid transporter in the small intestine. The elevated FGF15/19 proteins go back to the liver via portal vein and bind to the fibroblast growth factor receptor 4 (FGFR4) of hepatocytes. Upon activation of FGFR4, c-Jun NH(2)-terminal kinase (JNK) signaling pathway in turn suppresses CYP7A1 gene expression to reduce bile acid synthesis in the liver (Fig. 3).18192021
Generation of FXR-null mice has shown impaired glucose tolerance and insulin resistance, suggesting that FXR plays a pivotal role in glucose homeostasis. FXR is able to control gene expression of phosphoenolpyruvate carboxykinase (PEPCK), which is located in the liver to catalyze a crucial step of hepatic gluconeogenesis.62223 Besides PEPCK, FXR activation represses gene expression of glucose-6-phosphatase (G6pc), which catalyzes the hydrolysis of glucose-6-phosphate to glucose. In addition, FXR activation also increases glycogen levels in the liver as via enhancing downstream insulin receptor signaling.623
FXR-null mice studies further revealed the physiological roles of FXR to regulate lipid metabolism.24 Compared with littermate control, FXR-null mice exhibited elevated levels of plasma triglycerides and cholesterol.624 In addition, FXR-null mice also showed higher levels of lipoprotein (HDL) cholesterol in plasma than littermate control, which correlates with a reduction of hepatic expression of scanvenger receptor class B type I (SR-BI), a well-known receptor to clear HDL cholesterol from the blood.2526 Interestingly, transcriptional activation of FXR largely reduced plasma triglyceride levels in wild type mice24; FXR activation is able to suppress gene expressions involved in lipoprotein metabolism including sterol regulatory element-binding protein 1 transcription factor 1c (SREBP-1c), phospholipid transfer protein (PLTP), steroyl-CoA desaturase 1 (SCD-1), the very low density lipoprotein receptor (VLDLR), apolipoprotein C-II, and apolipoprotein E. Altogether, FXR is a key molecule to regulate glucose and lipid homeostasis.
Previous studies have shown that FXR plays a protective role to control intestinal inflammation to minimize bacterial overgrowth.27 With cholestasis model, bile duct ligation compromised FXR transcriptional activity in the intestine. Interestingly, bile duct ligation led to bacterial overgrowth in the small intestine and translocation into other peripheral tissues.27 FXR activation by synthetic FXR agonist treatment attenuated bacterial overgrowth and reduced intestinal inflammation. Furthermore, FXR has been shown to regulate expression of genes involved in epithelial barriers and antimicrobial peptides, including inducible nitric oxide synthase (iNOS), occluding, and interleukin-18 (IL-18).27 Thus, FXR activation reduces bacterial overgrowth by induction of genes involved in epithelial barriers and antimicrobial peptides.28
Besides intestinal inflammation, FXR has been reported to suppress colorectal tumorigenesis. Though bile acids including deoxycholic acid have been considered as stimulators of tumorigenesis in the colon, activation of FXR by synthetic FXR agonist largely suppressed intestinal tumorigenesis in Apc (Min/+) mice and colitis-associated colorectal cancer model.29 In case of FXR deficiency, Wnt signaling has been upregulated to promote basal proliferative activity in colonic epithelial cells.29 Thus, FXR activation would be useful in the treatment of colon cancer.
Recent study has shown that beneficial improvements by vertical sleeve gastrectomy (VSG) were diminished in FXR-null mice.30 These results reported that VSG in wild type mice increased circulating bile acids and changed gut microbial communities. Thus, FXR signaling is an important molecular underpinning for the beneficial effects of the VSG.30
In addition to the critical roles of FXR in VSG, a recent study has proposed therapeutic roles of FXR to protect against diet-induced obesity. By using gut-specific FXR synthetic agonist, named Fexaramine, intestinal specific FXR agonism largely increased the thermogenesis and mitochondrial oxidative phosphorylation in brown adipose tissues.31 The intestinal FXR agonism-mediated fat remodeling also led to increased browning of white adipose tissue. Thus, intestinal FXR agonism stimulated energy expenditure in brown and white adipose tissue, leading to increase whole body energy metabolism.31 The molecular mechanisms of how intestinal FXR agonism increased whole body energy metabolism remains still unclear.
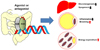
In contrast, another recent study has reported that ablation of intestine-specific FXR led to anti-obesity in obese animal models.32 As previously mentioned, FXR activation in the intestine is able to repress CYP7A1 expression in the liver, resulting in the reduction of bile acid synthesis. Ablation of intestine-specific FXR largely increased hepatic CYP7A1 gene expression and elevated circulating bile acid levels.32 Given that circulating bile acids are able to increase local thyroid signaling in brown adipose tissue and basal energy expenditure,33 intestine-specific FXR ablation resulted in anti-obesity phenotypes in animal models. Furthermore, inhibition of FXR activity in the intestine led to remodeling of gut microbiome to protect against diet-induced obesity.32 Together, both recent studies have suggested and demonstrated that regulation of intestinal FXR transcriptional activity would be a novel therapeutic strategy to protect against metabolic syndromes (Fig. 4).
Though bariatric surgery has been well known to improve metabolic parameters in the patients, the molecular mechanisms for beneficial effects still remain unclear. As reported, patients with bariatric surgery usually exhibit elevated circulating bile acid levels, FGF19, and Glucagon-like peptide 1 (GLP-1).343536 Given that bile acids are the endogenous ligand for nuclear receptor FXR, the physiological roles of FXR has been widely accepted as a novel therapeutic target for metabolic syndromes. Though numerous studies have revealed various physiological functions of FXR in the animal models of metabolic diseases, the development of synthetic modulator to regulate FXR transcriptional activities still needs to be required. Since FXR has been reported as a critical molecule to improve beneficial metabolic parameter in VSG using animal model, development of novel synthetic modulator for FXR would be useful therapeutic target for metabolic diseases.
Figures and Tables
Fig. 2
Regulation of cholesterol/bile acid metabolism by FXR. CYP7A1; Cholesterol-7α-hydroxylase, SHP; Small heterodimer partner, RXR; Retinoid X receptor, FXR; Farnesoid X receptor.
ACKNOWLEDGEMENT
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A01052195).
References
1. Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003; 72:137–174.

2. Tu H, Okamoto AY, Shan B. FXR, a bile acid receptor and biological sensor. Trends Cardiovasc Med. 2000; 10:30–35.

3. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999; 284:1362–1365.

4. Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995; 81:687–693.

5. Chiang JY. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev. 2002; 23:443–463.

6. Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006; 31:572–580.

7. Edwards PA, Kast HR, Anisfeld AM. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res. 2002; 43:2–12.

8. Liu J, Lu H, Lu YF, Lei X, Cui JY, Ellis E, et al. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol Sci. 2014; 141:538–546.

9. Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010; 8:e005.

10. Fujita K, Iguchi Y, Une M, Watanabe S. Ursodeoxycholic acid suppresses lipogenesis in mouse liver: possible role of the decrease in beta-muricholic acid, a farnesoid X receptor antagonist. Lipids. 2017; 52:335–344.

11. Jones DE. Obeticholic acid for the treatment of primary biliary cirrhosis. Expert Rev Gastroenterol Hepatol. 2016; 2:1–9.

12. Thomas H. Therapy: obeticholic acid for PBC. Nat Rev Gastroenterol Hepatol. 2016; 13:558–559.
14. Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res. 2006; 47:201–214.

15. Li-Hawkins J, Gåfvels M, Olin M, Lund EG, Andersson U, Schuster G, et al. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. 2002; 110:1191–1200.

16. Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000; 6:507–515.

17. Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, et al. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002; 2:721–731.

18. Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis. 2015; 33:327–331.

19. Stroeve JH, Brufau G, Stellaard F, Gonzalez FJ, Staels B, Kuipers F. Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice. Lab Invest. 2010; 90:1457–1467.

20. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005; 2:217–225.

21. Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009; 49:297–305.

22. Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, Christe ME, et al. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 2005; 146:984–991.

23. Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006; 116:1102–1109.

24. Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006; 103:1006–1011.

25. Zhang Y, Yin L, Anderson J, Ma H, Gonzalez FJ, Willson TM, et al. Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia. J Biol Chem. 2010; 285:3035–3043.

26. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000; 102:731–744.

27. Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006; 103:3920–3925.

28. Zhou X, Cao L, Jiang C, Xie Y, Cheng X, Krausz KW, et al. PPARalpha-UGT axis activation represses intestinal FXR-FGF15 feedback signalling and exacerbates experimental colitis. Nat Commun. 2014; 5:4573.

29. Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008; 68:9589–9594.

30. Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014; 509:183–188.

31. Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015; 21:159–165.

32. Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013; 4:2384.

33. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006; 439:484–489.

34. Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013; 36:1859–1864.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download