Abstract
Objective
Black raspberry (Rubus occidentalis) has been known for its anti-inflammatory and anti-oxidant effects and for improving vascular endothelial function in patients at high-risk for cardiovascular disease. We investigated short-term effects of black raspberry on lipid profiles, vascular endothelial function and circulating endothelial progenitor cells in statin naïve participants with metabolic syndrome.
Methods
Patients with metabolic syndrome (n=51) without lipid lowering medications were prospectively randomized into the black raspberry group (n=26, 750 mg/day) and placebo group (n=25) during the 12-week follow-up. Lipid profiles, brachial artery flow-mediated dilatation (baFMD) and inflammatory cytokines such as IL-6, TNF-α, C-reactive protein, adiponectin, sICAM-1, sVCAM-1 were measured at baseline and at 12-week follow-up. Central blood pressure and augmentation index were also measured at baseline and at 12-week follow-up.
Results
Decreases from baseline in total cholesterol levels (-22.7±34.3 mg/dL vs. 0.0±34.7mg/dL, p<0.05, respectively) and total cholesterol/HDL ratio (-0.34±0.68 vs. 0.17±0.56, p<0.05, respectively) were significantly greater in the black raspberry group when compared to the placebo group. Decreases from baseline in IL-6 (-0.5±1.4 pg/mL vs. -0.1±1.1 pg/mL, p<0.05, respectively) and TNF-α levels (-5.4±4.5 pg/mL vs. -0.8±4.0 pg/mL, p<0.05, respectively) were significantly greater in the black raspberry group. Increases from the baseline in adiponectin levels (2.9±2.1 µg/mL vs. -0.2±2.5 µg/mL, p<.05) were significant in the black raspberry group. Increases in baFMD at 12-week follow-up were significantly greater in the black raspberry group when compared to the placebo group (2.9±3.6 mm vs. 1.0±3.9 mm, p<0.05, respectively). Radial augmentation indexes were significantly decreased in the black raspberry group when compared to the placebo group (-2±10% vs. 4±13%, p<0.05).
Figures and Tables
Table 1
Baseline patient characteristics

Table 2
Changes in lipid profiles
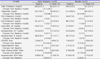
Table 3
Changes in inflammatory parameters during the 12-week follow-up

Table 4
Changes in flow-mediated dilation during the 12-week follow-up

Table 5
Changes in baPWV, carotid IMT and ankle brachial index during the 12-week follow-up

Table 6
Changes in central blood pressure and radial augmentation index

Table 7
Changes in circulating endothelial progenitor cells during the 12-week follow-up

References
1. Kim EJ, Lee YJ, Shin HK, Park JH. Induction of apoptosis by the aqueous extract of Rubus coreanum in HT-29 human colon cancer cells. Nutrition. 2005; 21:1141–1148.

2. Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem. 2000; 48:140–146.

3. Lim JW, Hwang HJ, Shin CS. Polyphenol compounds and anti-inflammatory activities of Korean black raspberry (Rubus coreanus Miquel) wines produced from juice supplemented with pulp and seed. J Agric Food Chem. 2012; 60:5121–5127.

4. Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002; 106:1652–1658.

5. Zhao C, Kim HK, Kim SZ, Chae HJ, Cui WS, Lee SW, et al. What is the role of unripe Rubus coreanus extract on penile erection? Phytother Res. 2011; 25:1046–1053.

6. Yang HM, Oh SM, Lim SS, Shin HK, Oh YS, Kim JK. Antiinflammatory activities of Rubus coreanus depend on the degree of fruit ripening. Phytother Res. 2008; 22:102–107.

7. Ou HC, Chou FP, Sheen HM, Lin TM, Yang CH, Huey-Herng Sheu W. Resveratrol, a polyphenolic compound in red wine, protects against oxidized LDL-induced cytotoxicity in endothelial cells. Clin Chim Acta. 2006; 364:196–204.

8. Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996; 27:363–366.

9. Rakici O, Kiziltepe U, Coskun B, Aslamaci S, Akar F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int J Cardiol. 2005; 105:209–215.

11. McAnulty LS, Collier SR, Landram MJ, Whittaker DS, Isaacs SE, Klemka JM, et al. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr Res. 2014; 34:577–584.

12. Ash MM, Wolford KA, Carden TJ, Hwang KT, Carr TP. Unrefined and refined black raspberry seed oils significantly lower triglycerides and moderately affect cholesterol metabolism in male Syrian hamsters. J Med Food. 2011; 14:1032–1038.

13. Lee SM, Choi Y, Sung J, Kim Y, Jeong HS, Lee J. Protective effects of black rice extracts on oxidative stress induced by tert-butyl hydroperoxide in hepg2 cells. Prev Nutr Food Sci. 2014; 19:348–352.

14. Jeong HS, Kim S, Hong SJ, Choi SC, Choi JH, Kim JH, et al. Black raspberry extract increased circulating endothelial progenitor cells and improved arterial stiffness in patients with metabolic syndrome: a randomized controlled trial. J Med Food. 2016; 19:346–352.

15. Jeong HS, Hong SJ, Cho JY, Lee TB, Kwon JW, Joo HJ, et al. Effects of Rubus occidentalis extract on blood pressure in patients with prehypertension: randomized, double-blinded, placebo-controlled clinical trial. Nutrition. 2016; 32:461–467.

16. Jeong HS, Hong SJ, Lee TB, Kwon JW, Jeong JT, Joo HJ, et al. Effects of black raspberry on lipid profiles and vascular endothelial function in patients with metabolic syndrome. Phytother Res. 2014; 28:1492–1498.

17. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992; 340:1111–1115.

18. Wang SY, Jiao H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem. 2000; 48:5677–5684.

19. Seeram NP, Nair MG. Inhibition of lipid peroxidation and structure-activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J Agric Food Chem. 2002; 50:5308–5312.

20. Viljanen K, Kylli P, Kivikari R, Heinonen M. Inhibition of protein and lipid oxidation in liposomes by berry phenolics. J Agric Food Chem. 2004; 52:7419–7424.

21. Shafiee M, Carbonneau MA, Urban N, Descomps B, Leger CL. Grape and grape seed extract capacities at protecting LDL against oxidation generated by Cu2+, AAPH or SIN-1 and at decreasing superoxide THP-1 cell production. A comparison to other extracts or compounds. Free Radic Res. 2003; 37:573–584.

22. Demrow HS, Slane PR, Folts JD. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995; 91:1182–1188.

23. Vivancos M, Moreno JJ. Effect of resveratrol, tyrosol and beta-sitosterol on oxidised low-density lipoprotein-stimulated oxidative stress, arachidonic acid release and prostaglandin E2 synthesis by RAW 264.7 macrophages. Br J Nutr. 2008; 99:1199–1207.

24. Bhandary B, Lee GH, So BO, Kim SY, Kim MG, Kwon JW, et al. Rubus coreanus inhibits oxidized-LDL uptake by macrophages through regulation of JNK activation. Am J Chin Med. 2012; 40:967–978.

25. Nakanishi T, Mukai K, Yumoto H, Hirao K, Hosokawa Y, Matsuo T. Anti-inflammatory effect of catechin on cultured human dental pulp cells affected by bacteriaderived factors. Eur J Oral Sci. 2010; 118:145–150.

26. Ndiaye M, Chataigneau T, Andriantsitohaina R, Stoclet JC, Schini-Kerth VB. Red wine polyphenols cause endothelium-dependent EDHF-mediated relaxations in porcine coronary arteries via a redox-sensitive mechanism. Biochem Biophys Res Commun. 2003; 310:371–377.

27. Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999; 100:1050–1055.

28. Karim M, McCormick K, Kappagoda CT. Effects of cocoa extracts on endothelium-dependent relaxation. J Nutr. 2000; 130:2105S–2108S.

29. Basu A, Fu DX, Wilkinson M, Simmons B, Wu M, Betts NM, et al. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr Res. 2010; 30:462–469.

30. Graf D, Seifert S, Jaudszus A, Bub A, Watzl B. Anthocyanin-rich juice lowers serum cholesterol, leptin, and resistin and improves plasma fatty acid composition in fischer rats. PLoS One. 2013; 8:e66690.

31. Garcia-Diaz DF, Johnson MH, de Mejia EG. Anthocyanins from fermented berry beverages inhibit inflammationrelated adiposity response in vitro. J Med Food. 2015; 18:489–496.

32. Suzuki A, Yamamoto N, Jokura H, Yamamoto M, Fujii A, Tokimitsu I, et al. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J Hypertens. 2006; 24:1065–1073.

33. Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, Chowienczyk P, et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am J Clin Nutr. 2012; 96:781–788.

34. Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007; 85:895–909.

35. Nagasawa SY, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, et al. Relation between serum total cholesterol level and cardiovascular disease stratified by sex and age group: a pooled analysis of 65 594 individuals from 10 cohort studies in Japan. J Am Heart Assoc. 2012; 1:e001974.
36. Lawrence T, Willoughby DA, Gilroy DW. Anti- inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002; 2:787–795.

37. Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005; 208:249–258.

38. Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004; 84:1381–1478.

39. Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006; 113:2135–2150.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download