Abstract
Objective
Atrial fibrillation (AF) is a common arrhythmia which is generally more prevalent in the elderly population. However, the new onset of AF is frequently found in young populations. In order to identify putative prognostic biomarkers for detection of young-onset AF, we purified and characterized lipoproteins in terms of oxidative and inflammatory properties.
Methods
Male patients with AF (46±7 years of age, n=19) were recruited. Their serum and individual lipoproteins were analyzed and compared with healthy controls (48±9 years of age, n=17).
Results
The patients with AF revealed hypertriglyceridemia, hyperuricemia with mild obesity, elevated levels of CRP, and a normal level of cholesterol. All lipoproteins from patients with AF demonstrated higher levels of TG and advanced glycated end products, and decreased particle size than controls. AF-LDL showed an increased extent of oxidation with increased atherogenic macrophage phagocytosis. AF-HDL showed impaired antioxidant ability and a lower level of apoA-I expression.
Atrial fibrillation (AF) is the most common arrhythmia in adults. An increased prevalence of AF is anticipated with the increase in the aging population and survivability of patients with chronic heart disease.1 A growing body of evidence implicates oxidative stress in the pathogenesis and perpetuation of AF.234 These findings were primarily derived from studies of animal and human samples with persistent or permanent forms of AF.
It has been well known that increased inflammation promotes persistent AF. Oxidative stress and endothelial dysfunction have also been associated with the incidence of AF.5 Serum lipoprotein, especially very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL), is susceptible to oxidative and inflammatory stress. High-density lipoproteins (HDL) exerts many beneficial effects for the maintenance of a healthy physiologic system, including antioxidant, anti-inflammatory, and anti-thrombotic effects.67 These activities are exerted in accordance with the composition of essential apolipoproteins and associated enzymes. Biochemical modifications that affect HDL structure and function,8 include oxidation and non-enzymatic glycation to produce advanced glycated end products (AGEs) via the Maillard reaction.9 Although it has been speculated that patients with cardiovascular disease showed impaired lipoprotein functionality, however, there has been no direct evidences were reported about feature of lipoproteins in regarding structural and functional correlations.
This study was designed to compare individual lipoprotein properties between male patients with AF and controls with similar age to provide distinct biomarkers in apolipoprotein and lipoprotein metabolism. In order to identify unique properties in lipoprotein levels regarding oxidation and inflammation, we purified and analyzed lipoproteins from the sera of middle aged patients with AF in lipid and protein compositions and enzymatic function.
We recruited male patients with AF (n=19; 46±7 years old) and male controls (n=17, 48±9 years old) with similar age. Heavy alcohol consumers (>30 g EtOH/day) and those who had consumed any prescribed drugs to treat hyperlipidemia, diabetes mellitus, or hypertension were excluded. All subjects had unremarkable medical records without histories of endocrinologic disorders. Informed consent was obtained from all patient and subjects prior to enrollment in the study and the Institutional Review Board at the Medical Center of Yeungnam University (Daegu, South Korea) approved the protocol.
Blood was drawn on overnight fasting state in AF patients and control. Blood was collected using a vacutainer (BD sciences, Franklin Lakes, NJ) containing EDTA (final 1 mM). Plasma was isolated by low speed centrifugation and stored at -80℃ until analysis. Blood parameters, lipids, and glucose concentrations were determined using an automatic blood analyzer (Chemistry analyzer AU4500 Olympus, Tokyo, Japan).
VLDL (d<1.019 g/mL), LDL (1.019<d<1.063), HDL2 (1.063<d<1.1α25), and HDL3 (1.125<d<1.225) were isolated from individual patient and control sera via sequential ultracentrifugation,10 with the density adjusted by the addition of NaCl and NaBr in accordance with standard protocols. Samples were centrifuged for 24 hours at 10℃ at 100,000xg using a Himac CP-90α (Hitachi, Tokyo, Japan) at the Instrumental Analysis Center of Yeungnam University.
For each of the lipoproteins which were individually purified, total cholesterol (TC) and triglyceride (TG) measurements were obtained using commercially available kits (cholesterol, T-CHO, and TG, Cleantech TS-S; Wako Pure Chemical, Osaka, Japan). The protein concentrations of lipoproteins were determined via the Lowry protein assay, as modified by Markwell et al.11 using the Bradford assay reagent (BioRad, Seoul, South Korea) with bovine serum albumin (BSA) as a standard. To assess the degree of oxidation of individual LDL, the concentration of oxidized species in LDL was determined by the thiobarbituric acid reactive substances (TBARS) method using malondialdehyde as a standard.12
In order to compare the extent of glycation between the groups, the content of AGEs in the individual lipoproteins were determined from reading the fluorometric intensities at 370 nm (excitation) and 440 nm (emission), as described previously.13
To compare the susceptibility of copper-mediated LDL oxidation, 300 µg of LDL was incubated with 5 µM CuSO4 for up to 3 hours. During the incubation, the quantity of formed conjugated dienes was monitored by measuring the absorbance at 234 nm (Abs234) at 37℃14 using a Beckman DU 800 spectrophotometer equipped with a MultiTemp III thermocirculator.
In order to verify the spectroscopic data, the oxLDL samples were subjected to electrophoresis on a 0.5% agarose gel for an electromobility comparison.15 The post-oxidative electrophoretic mobility of LDL was compared via electrophoresis on a 0.5% agarose gel because there is some modification of amino acids in the apo-B by the oxidation.
The ferric reducing ability of plasma (FRAP) was determined using the method described by Benzie and Strain16 with a slight modification, as described recently by our research group.17 The antioxidant activities of the individual HDL fractions (20 µg each) were then estimated by measuring the increase in absorbance induced by the generated ferrous ions.
A rHDL-containing apoA-I and cholesteryl oleate was synthesized in accordance with the method described by Cho et al.1819 using trace amounts of [3H]-cholesteryl oleate (TRK886, 3.5 µCi/mg of apoA-I; GE Healthcare). The CE-transfer reaction was allowed in 300 µL reaction mixtures that contained equal amounts of the individual lipoproteins (20 µL, 10-20 µg of protein ) as a Colesteryl Ester Transfer Protein (CETP) source, and rHDL-agarose (50 µL, 0.25 mg/mL) and human LDL (50 µL, 0.25 mg/mL) as a CE-donor and CE-acceptor, respectively. After incubation at 37℃, the reaction was halted via brief centrifugation (10,000xg) for 3 minutes at 4℃. The supernatant (150 µL) was then subjected to scintillation counting, and the percentage transfer of [3H]-CE from rHDL to LDL was calculated.
Paraoxonase-1 (PON-1) activity toward paraoxon was determined by evaluating the hydrolysis of paraoxon into p-nitrophenol and diethylphosphate, which was catalyzed by the enzyme.20 PON-1 activity was then determined by measuring the initial velocity of p-nitrophenol production at 37℃, as determined by measuring the absorbance at 415 nm (microplate reader, Bio-Rad model 680; Bio-Rad, Hercules, CA, USA), as described previously.21
The apolipoprotein/lipoprotein compositions were compared via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with equal protein loading quantities (3 µg of total protein per lane) from individual HDL3, and the levels of expression of apolipoprotein were analyzed via immunodetection. Anti-human apoA-I antibody (ab7613) was purchased from Abcam (Cambridge, UK). The relative band intensity (BI) was compared via band scanning with Gel Doc® XR (Bio-Rad) using Quantity One software (version 4.5.2).
As shown in Table 1, the AF group had slightly higher obese body mass index (27±4 kg/m2) than that of control group (24.7±3 kg/m2) although the blood pressures were in the normal range around 125 mmHg and 75 mmHg for systolic and diastolic blood pressure, respectively. Interestingly, the AF group showed 12% lower serum TC and 15% lower HDL-C than control group. However, AF group showed 1.5-fold elevated serum TG compared with control and TG/HDL ratio in AF group was also increased upto 1.8-fold higher than control group. TC/HDL and LDL/HDL ratios were similar between the groups (4.2-4.1 and 3.0-2.6, respectively). Thyroid stimulating hormone is elevated in the AF group, although there was no difference. The serum glucose level was elevated 19% in the AF group compared to the control group, although it was fall under the normal blood glucose range (<110 mg/dL). AF group had a normal creatinine and blood urea nitrogen levels, while the uric acid level was elevated 40% in the AF group compared to the control group. The AF group had a significantly elevated hsCRP level (2-fold higher than the control group), while hepatic inflammatory parameters (AST and ALT) were in the normal range.
Under the same protein concentration, the cholesterol content in VLDL were similar between the groups, however, TG was 1.5-fold more enriched in AF group. In LDL, cholesterol content was 13% reduced in AF group, while TG content was 2-fold elevated. In HDL2, cholesterol content was slightly reduced and TG content was 2-fold elevated in AF group. In HDL3, the TG content in AF group elevated 2-fold more than control, while cholesterol contents were similar between the group.
All lipoproteins from the AF group contained more oxidized species by conjugated diene determination (A234), as shown in Fig. 2A and TBARS quantification with the MDA standard (Fig. 2B). From conjugated diene determination, VLDL in AF group showed the highest extent of oxidation, while LDL and HDL from AF group showed slightly higher than control. TBARS assay showed AF group showed higher MDA content in all lipoproteins especially in LDL, 5-fold higher than control.
As shown in Fig. 2C, without cupric ion treatment, the conjugated diene level (A234) of AF-LDL was increased after an 80 min incubation at 37℃, while the control-LDL did not show an increase of A234. Treatment with cupric ion accelerated more increase of A234 in the AF-LDL group after 40 min, while the control-LDL showed an increase of A234 after 60 min. AF-LDL was more sensitive to oxidation under with or without cupric ion. Electrophoresis revealed that AF-LDL was more sensitive to the cupric ion-mediated oxidation with time dependent manner since it migrated faster than the control-LDL as indicated by arrow (Fig. 2D).
The AF group showed weaker HDL3-associated PON activity than the control, while HDL2-associated PON activity was not detected in both groups (Fig. 3A). Ferric ion removal ability was also lowered in AF-HDL3 (Fig. 3B). Electrophoretic analysis (15% SDS-PAGE) with HDL2 revealed that no major different band patterns were found between the groups (Fig. 4A). While the level of apoA-I expression in HDL3 was not different between the groups, the AF group had a 10% lower level of expression of apoA-I in HDL2 from Western blot analysis (Fig. 4B). The AF group exhibited a 21% higher level of expression of the paraoxonase level in HDL3 as shown in Fig. 4B.
After a 48-hour incubation, treatment AF-VLDL (16 µg of protein) or AF-LDL (30 µg of protein) resulted in much stronger red intensity than the same amount of control VLDL and LDL, as shown in photo of Fig. 5. This result indicates that VLDL and LDL from the AF group could be more easily taken up into macrophages by phagocytosis. Determination of oxidized species with the culture media revealed that AF-VLDL- and AF-LDL-treated media contained 40% and 23% more oxidized species, respectively, as shown in graph of Fig. 5.
AF and chronic heart failure are major cardiovascular disorders (CVD) which share common risk factors and pathophysiologic processes in serum and lipoprotein.
A number of reports have suggested that the incidence of CVD is highly dependent on the properties and qualities of lipoprotein. In the current study, we compared structural and functional properties of lipoproteins, especially with respect to the extent of oxidation and the antioxidant ability of individual lipoproteins to find putative biomarkers or risk factors in male patients with AF. Although the risk of AF is markedly increased with advancing age,2223 the patients in this study were relatively young adults (46±7 years of age).
Many cardiovascular diseases are associated with dyslipidemia, and lipid testing and treatment are well-established clinical tools to treat ischemic heart disease. Although the relationship of dyslipidemia and AF has not been firmly established, other studies as well as the current study showed that AF patients have normal serum cholesterol levels.24 In this study, however, the young male patients had hypertriglyceridemia and hyperuricemia. However, the serum TG level is an independent risk factor of coronary artery disease. In male CVD patients, we and others have suggested that the TG-rich lipoproteins are pro-atherogenic2526 and more specific for myocardial infarction. The presence of increased TG in lipoprotein is in good agreement with the results of a previous study that indicated that TG levels are an important and independent predictor of CAD and stroke risk in the Asia-Pacific region.27 In this report, serum TG and TG-enriched lipoprotein was highly associated with AF, but apoC-III was not elevated in the HDL fraction (Fig. 4A).
On the other hand, an elevated serum uric acid level has long been recognized as a common feature in patients with metabolic syndrome (MetS).28 Nicolaou et al.29 suggested that MetS patients who had paroxysmal AF had increased cardiovascular mortality with elevation of uric acid (6.2±1.0 mg/dL). In addition, Karagiannis et al.30 reported that an elevated level of serum uric acid level is independently associated with an increased risk of early death in patients with acute stroke. Taken together, elevated serum TG and uric acid levels are possibly important and potentially modifiable risk factors for AF.
The pathophysiologic mechanism of AF is closely related to an increase in oxidative stress and inflammatory processes in the blood.31 Several inflammatory factors are known as risk factors for AF, including CRP and osteoprotegerin, although the relevant physiologic role is still under investigation. Increased oxidized LDL is a known risk factor of CVD and HDL-associated antioxidant enzymes are inversely associated with the incidence of CVD. Oxidative modification of LDL is an important feature of atherosclerosis. A greater extent of oxLDL is more easily taken up by macrophage scavenger receptors with high efficiency and leads to cholesterol loading in macrophages and foam cell formation, as shown in Fig. 5.
Consumption of fish32 and n-3 polyunsaturated fatty acid (PUFA)33 are associated with a lower incidence of AF. These results suggest that the elevated antioxidant potential of serum influence the incidence of AF. Similarly, Calabresi34 reported that high intake of PUFA increases plasma PON. PON has emerged as the component of HDL most likely to explain its ability to attenuate the oxidation of LDL; PON might be a major defense barrier against lipid peroxides from oxLDL. Tomas et al.35 reported that high oleic acid intake is associated with significantly increased HDL-cholesterol concentrations and PON activity.
In conclusion, the young male patients with AF in the present study had elevated levels of serum TG, uric acid, and CRP, with normal levels of cholesterol. All lipoproteins from AF had more elevated levels of TG and advanced glycated end products, and decreased particle size than controls. AF-LDL had a greater extent of oxidation and were more atherogenic in macrophage phagocytosis. AF-HDL showed impaired antioxidant ability and a lower level of expression of apoA-I. These data suggest that lipoprotein properties are severely modified in young patients with AF, which is correlated with increased oxidation and inflammation.
Figures and Tables
 | Fig. 1Antioxidant ability of serum. (A) Serum paraoxonase activity. Twenty microliters of equally diluted serum (10 mg/mL) was added to 230µL of the paraoxon-ethyl (Sigma catalog# D-9286) solution containing 90 mM Tris-HCl, 3.6 mM NaCl, and 2 mM CaCl2 (pH 8.5) for 30 min. Error bars indicate the SD from three independent experiments with duplicate samples, (B) Ferric ion removal ability. Rate of increase in absorbance at 593 nm. The HDL3 of control group showed much weaker reducing ability than the HDL3 of control groups during 20 minutes of incubation. |
 | Fig. 2Comparison of oxidized extent in lipoprotein between the AF and control. (A) Quantification of conjugated diene level in lipoproteins under the same protein amount (VLDL, 3µg; LDL, 6µg; HDL2, 6µg; HDL3, 10 mg), (B) Determination of thiobarbituric acid reactive substances between AF and control groups using malondialdehyde standard. The same amount of protein was used. VLDL, 0.15 mg/mL; LDL, 0.33 mg/mL, (C) Monitoring of conjugated diene level production in LDL (0.3 mg/mL) under presence of Cu2+ (final 10µM), (D) Electromobility of LDL from the AF and controls with or without treatment of copper (final 10µM) for 6 hrs (0.5% agarose gel) |
 | Fig. 3Quantification of advanced glycated end products. The extent of glycation was determined from fluorescence (Ex=370 nm, Em=440 nm) measurement in each lipoprotein fraction using the same amount of protein. VLDL, 15µg; LDL, 32µg; HDL2, 30µg; HDL3, 50µg. |
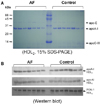 | Fig. 4Expressional profiles of apolipoproteins in HDL. (A) Electrophoretic patterns of HDL2 from AF and control group (15% SDS-PAGE). Purified HDL2 via ultracentrifugation was equally diluted and the same amount of protein (3µg) was loaded per lane and visualized by Coomassie blue staining, (B) mmunodetection of apoA-I in HDL2 and HDL3. Western blotting analysis with apoA-I antibody, which was raised by full-length apoA-I (ab7613; Abcam). |
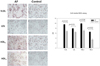 | Fig. 5Cellular uptake of VLDL and LDL into macrophage cell. PMA differentiated THP-1 cells were incubated with 50µL of VLDL (0.15 mg/mL) or LDL (0.33 mg/mL) from either AF or control group under presence of 450µL of RPMI1640 media. The extent of cellular uptake of the lipoproteins by macrophages was then compared by oil red O staining as described in the text. The cells were then photographed using a Nikon Eclipse TE2000 microscope (Tokyo, Japan) at 400× magnification. |
Table 1
Serum profiles of male AF patients and controls

BMI; body mass index, CETP; cholesteryl ester transfer protein, DBP; diastolic blood pressure, GOT; glutamate oxaloacetate transaminase, GPT; glutamate pyruvate transaminase, HDL-cholesterol; high-density lipoprotein-cholesterol, LDL-cholesterol, low-density lipoprotein-cholesterol, SBP; systolic blood pressure, TC; total cholesterol, TG; triglyceride, TSH; thyroid stimulating hormone
ACKNOWLEDGMENTS
This work was supported by a grant from the Mid-carrier Researcher Program (2014-11049455) and Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation (NRF), funded by the Ministry of Science, ICT and Future Planning of Korea.
References
1. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998; 82:2N–9N.
2. Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003; 108:3006–3010.

3. Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001; 104:2886–2891.

4. Gaudino M, Andreotti F, Zamparelli R, Di Castelnuovo A, Nasso G, Burzotta F, et al. The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication. Circulation. 2003; 108:Suppl 1. II195–II199.

5. Tousoulis D, Zisimos K, Antoniades C, Stefanadi E, Siasos G, Tsioufis C, et al. Oxidative stress and inflammatory process in patients with atrial fibrillation: the role of left atrium distension. Int J Cardiol. 2009; 136:258–262.

6. Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004; 95:764–772.

7. Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005; 96:1221–1232.

8. Cho KH. Biomedicinal implications of high-density lipoprotein: its composition, structure, functions, and clinical applications. BMB Rep. 2009; 42:393–400.

9. Pongor S, Ulrich PC, Bencsath FA, Cerami A. Aging of proteins: isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc Natl Acad Sci U S A. 1984; 81:2684–2688.

10. Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955; 34:1345–1353.

11. Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978; 87:206–210.

12. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958; 181:1199–1200.

13. McPherson JD, Shilton BH, Walton DJ. Role of fructose in glycation and cross-linking of proteins. Biochemistry. 1988; 27:1901–1907.

14. Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989; 6:67–75.

15. Noble RP. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968; 9:693–700.

16. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996; 239:70–76.

17. Park KH, Shin DG, Kim JR, Cho KH. Senescence-related truncation and multimerization of apolipoprotein A-I in high-density lipoprotein with an elevated level of advanced glycated end products and cholesteryl ester transfer activity. J Gerontol A Biol Sci Med Sci. 2010; 65:600–610.

18. Cho KH. Synthesis of reconstituted high density lipoprotein (rHDL) containing apoA-I and apoC-III: the functional role of apoC-III in rHDL. Mol Cells. 2009; 27:291–297.

19. Cho KH, Shin DG, Baek SH, Kim JR. Myocardial infarction patients show altered lipoprotein properties and functions when compared with stable angina pectoris patients. Exp Mol Med. 2009; 41:67–76.

20. Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983; 35:1126–1138.
21. Park KH, Shin DG, Kim JR, Hong JH, Cho KH. The functional and compositional properties of lipoproteins are altered in patients with metabolic syndrome with increased cholesteryl ester transfer protein activity. Int J Mol Med. 2010; 25:129–136.

22. Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol. 1994; 74:236–241.

23. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997; 96:2455–2461.

24. Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol. 1994; 74:236–241.

26. Libby P. Fat fuels the flame: triglyceride-rich lipoproteins and arterial inflammation. Circ Res. 2007; 100:299–301.
27. Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Whitlock G, et al. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation. 2004; 110:2678–2686.

28. Brodov Y, Behar S, Boyko V, Chouraqui P. Effect of the metabolic syndrome and hyperuricemia on outcome in patients with coronary artery disease (from the Bezafibrate Infarction Prevention Study). Am J Cardiol. 2010; 106:1717–1720.

29. Nicolaou VN, Papadakis JE, Karatzis EN, Dermitzaki SI, Tsakiris AK, Skoufas PD. Impact of the metabolic syndrome on atrial size in patients with new-onset atrial fibrillation. Angiology. 2007; 58:21–25.

30. Karagiannis A, Mikhailidis DP, Tziomalos K, Sileli M, Savvatianos S, Kakafika A, et al. Serum uric acid as an independent predictor of early death after acute stroke. Circ J. 2007; 71:1120–1127.

31. Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003; 108:3006–3010.

32. Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004; 110:368–373.

33. Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation. 2009; 120:2315–2321.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download