Abstract
A 43-year-old male presented with effort angina. Ammonia positron emission tomography (PET) revealed reversible perfusion defect in left anterior descending artery (LAD) and left circumflex artery (LCX) territories with decreased coronary flow reserve. Coronary angiogram showed significant stenosis in proximal LAD and intermediate diffuse stenosis in LCX and right coronary artery (RCA). Fractional flow reserve (FFR) showed similar results with ammonia PET. After percutaneous coronary intervention for LAD and LCX, flow and pressure checked by PET and FFR showed improvement. Simultaneously use of ammonia PET and FFR could be useful for determining ischemia-inducible lesion especially in diffuse intermediate lesion with discrepancy between functional studies.
It is important to determine which vessel is responsible for ischemia in patients with multivessel coronary artery lesions.12 Functional study guided percutaneous coronary intervention (PCI) improved both symptom and prognosis in patients with multi-vessel disease.1 13N-ammonia positron emission tomography (PET) is a non-invasive method for measure reversible perfusion defect and viability using coronary flow reserve (CFR).3 Fractional flow reserve (FFR) is a technique to measure pressure differences between distal to stenosis and normal maximum flow in the same vessel.4 However, there are several discrepancies between flow predicted by CFR and pressure predicted by FFR especially in patients with diffuse coronary artery lesions.5
We report a case of multi-vessel coronary artery disease with diffuse intermediate lesions which performed 13N-ammonia PET and FFR simultaneously to estimate functional significance.
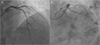
A 43-year-old male visited outpatient department due to Canadian Cardiovascular Society (CCS) class II angina which developed 2 months ago. His chest pain lasted for 5 minutes and was relieved by sublingual nitrate. Atherosclerotic risk factors were smoking and hypertension. His initial blood pressure was 130/90 mmHg with 75 beats per minute of heart rate. He was diagnosed as a stable angina. Initial 12-lead electrocardiogram showed normal sinus rhythm without ST-T changes, and transthoracic echocardiography showed no regional wall motion abnormality with normal Left ventricular (LV) function. 13N-ammonia positron emission tomography (PET) was done as a non-invasive stress test, and it showed reversible perfusion defects in anterior and lateral LV wall with decreased coronary flow reserve (Fig. 1). And then diagnostic coronary angiography was performed due to reversible large regional perfusion defect on PET study. It revealed significant stenosis in proximal left anterior descending artery (LAD), intermediate stenosis in distal right coronary artery (RCA) and proximal left circumflex artery (LCX) (Fig. 2). Fractional flow reserve (FFR) was checked to decide whether to revascularize intermediate stenosis in LCX and RCA. The values of FFR were 0.86 for RCA and 0.71 for LCX. FFR value can be changed in multiple lesions in single vessel. Although his coronary lesions were multivessel disease, each coronary artery had single stenotic lesion. The values of FFR for RCA and LCX were not changed in each coronary artery. Then, LCX and LAD lesions were sequentially revascularized with 3.0×30 mm and 3.5×34 mm drug-eluting stents (Resolute Integrity Stent®, Medtronic, Minneapolis, MN, USA), based on PET and FFR findings (Fig. 3). Post-procedural FFR value for LCX was 0.93 and follow-up 13N-Ammonia PET reconstruction images showed markedly improved myocardial perfusion in entire LV wall (Fig. 4). After successful PCI, his chest pain disappeared and he was discharged without any other in-hospital complications at the third hospital days. At a routine out-patient clinic follow-up, he did not complain of any symptoms after PCI.
PET is a recently developed noninvasive nuclear imaging method of evaluating myocardial perfusion and viability. It is able to measure perfusion and metabolism simultaneously with 13N-ammonia PET. The advantages of 13N-ammonia PET over single-photon emission computed tomography (SPECT) are superior resolution, higher sensitivity and specificity, lower radiation dose, ability to measure rest or stress perfusion status of each coronary artery and possibility of calculating absolute CFR.3 The short term prognostic value of 82Rb PET myocardial perfusion imaging (MPI) has been demonstrated.6 An added value of coronary flow reserve has been found as assessed by either 13N-ammonia7 or 82Rb.8 In addition, long-term prognostic value of 13N-ammonia PET has been demonstrated in a recent study.9 This case was 3 vessel disease. But, 13N-ammonia PET showed reversible perfusion defect with decreased CFR in anterior and lateral LV wall which was contributed by LAD and LCX. PET is a powerful tool in decision-making process of multivessel coronary artery lesions.
It is difficult to determine which lesions cause ischemia. Discrepancy between angiographic stenosis severity and functional significance in FFR existed about 36.3% of patients in Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) study. Patients with severe angiographic stenoses more than 70%, some of lesions are not related to myocardial ischemia.10 In multivessel coronary artery lesions, FFR-guided PCI could improve cardiovascular outcomes compared to conventional angiography-guided PCI.1 This case was proximal LAD lesion including 2 intermediate stenosis. Therefore we performed FFR to determine the need for revascularization. And then, PCI to LAD and LCX was done. We were able to avoid unnecessary PCI to RCA lesion.
Discordance between pressure-based FFR and flow-based CFR exist in 40% of patients.5 The 50% of discordance between FFR and CFR generally arise in diffuse long CAD. Diffuse narrowing of coronary artery reduces CFR considerably but with only a minimal decrease in segmental pressure gradient.3 The present case had diffuse long coronary lesions more than 20 mm. Although pre-PCI CFR measured by PET was decreased, FFR might be normal. The results of LCX pre-PCI FFR and CFR were decreased and consistent each other. Post procedural FFR value and CFR measured by PET were markedly improved.
This case is the first case of functional guided PCI using both FFR and CFR in patients with multivessel disease.
Figures and Tables
 | Fig. 1
13N-ammonia PET findings. (A) Reversible perfusion defect in anterior and lateral LV wall was revealed, (B) Coronary flow reserve was decreased in left anterior descending artery and left circumflex artery territory. |
 | Fig. 2Coronary angiography. (A) Left coronary angiogram reveals significant stenosis in proximal LAD, (B) Left coronary angiogram shows intermediate stenosis in proximal LCX, (C)(D) Right coronary angiogram shows intermediate stenosis in distal RCA. |
References
1. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010; 56:177–184.

2. Nam CW, Mangiacapra F, Entjes R, Chung IS, Sels JW, Tonino PA, et al. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol. 2011; 58:1211–1218.

3. Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013; 62:1639–1653.
4. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ, et al. Measurement of fractional flow reserve to assess the functional severity of coronaryartery stenoses. N Engl J Med. 1996; 334:1703–1708.

5. Johnson NP, Kirkeeide RL, Gould KL. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging. 2012; 5:193–202.

6. Yoshinaga K, Chow BJ, Williams K, Chen L, deKemp RA, Garrard L, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006; 48:1029–1039.

7. Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009; 54:150–156.

8. Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011; 58:740–748.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download